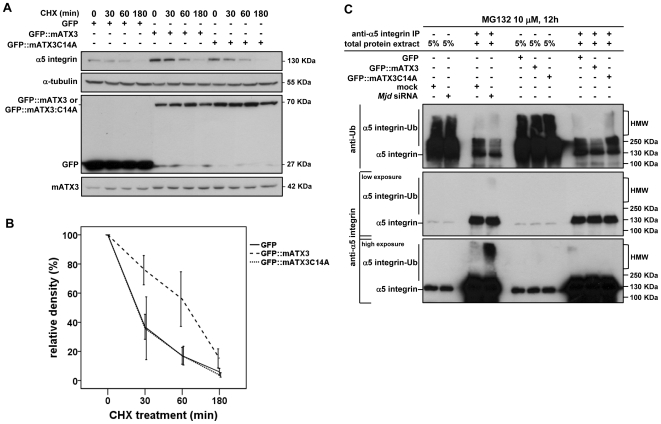
Figure 6. Ataxin-3 stabilizes α5 integrin subunit and represses its proteolysis.
(A) Effect of mATX3 overexpression in the half-life of α5 integrin subunit. C2C12 cells were transfected with the pEGFP:Mjd (GFP::mATX3), the pEGFP:MjdC14A (GFP::mATX3C14A) or the pEGFP-C1 (GFP)-empty plasmids, and after 48 hrs cells were treated with cycloheximide for 0, 30, 60 or 180 min. Whole cell extracts were collected for these time points and analyzed for the levels of endogenous α5 integrin subunit by immunoblotting. The overexpression of GFP::mATX3, but not the of GFP or the catalytic mutant GFP::mATX3C14A, was shown to stabilize the levels of α5 integrin subunit. (B) The graph represents the relative amounts of α5 integrin subunit in GFP, GFP::mATX3 and GFP::mATX3C14A overexpressing cells at various cycloheximide treatment times. The levels of endogenous mATX3 were similar in all the transfections. The results were normalized for α-tubulin and correspond to the average of three independent transfections ± SEM (error bars). (C) Denature-renature immunoprecipitation of α5 integrin subunit from Mjd siRNA, mock, GFP, GFP::mATX3, or GFP::mATX3C14A transfected C2C12 cells treated with the proteasome inhibitor MG132 at 10 µM, during 12 h. The immunoprecipitated forms of α5 integrin subunit were separated in a SDS-PAGE gel and blotted for ubiquitin and α5 integrin subunit (low and high exposure of the same blot). An increase of ubiquitinated forms of α5 integrin subunit was detected in mATX3-depleted cells. Cells overexpressing GFP::mATX3 showed a reduction in ubiquitinated α5 integrin subunit forms. No decrease of these species was observed in cells overexpressing the catalytic mutant GFP::mATX3C14A.