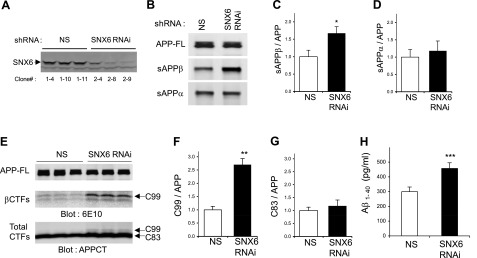
Figure 4.
Reducing SNX6 increases BACE1-mediated cleavage of APP. A) SNX6 expression in stable HEK293 clonal cell lines transfected with either nonsilencing shRNA vector (NS) or shRNA against human SNX6 (SNX6). B) Immunoprecipitation and Western blot analysis of sAPPβ and sAPPα, secreted from the HEK293 stable cells in A that were transfected with APP. At 1 d post-transfection, conditioned medium collected after 3 h of incubation was subjected to immunoprecipitation using either BACE1 cleavage site-specific antibody (sβwt) or 6E10, followed by Western blot analysis with LN27 to detect sAPPβ and sAPPα, respectively. C, D) Quantification of the sAPPβ (C) and sAPPα (D) bands (normalized to full-length APP) was performed using infrared-based quantitation (LI-COR) and combining >6 independent experiments. E) Detection of a major BACE1-derived C-terminal fragment (C99) and α-secretase-derived C83. HEK293 stable cells in A were transfected with APP. After 1 d of incubation with compound E to inhibit γ-secretase activity, cell lysates were subjected to Western blot analyses using indicated antibodies. F, G) Quantification of the C99 (F) and C83 (G) bands (normalized to full-length APP) was performed using infrared-based Western blot analysis (LI-COR) and combining 4 independent experiments. H) Reducing SNX6 increases the secretion of Aβ1–40. Conditioned medium was subjected to sandwich ELISA to quantify Aβ1–40 (n=3). Values are means ± sd. *P < 0.0005, **P < 0.00005, ***P < 0.005; 2-tailed t test.