Fig. 2.
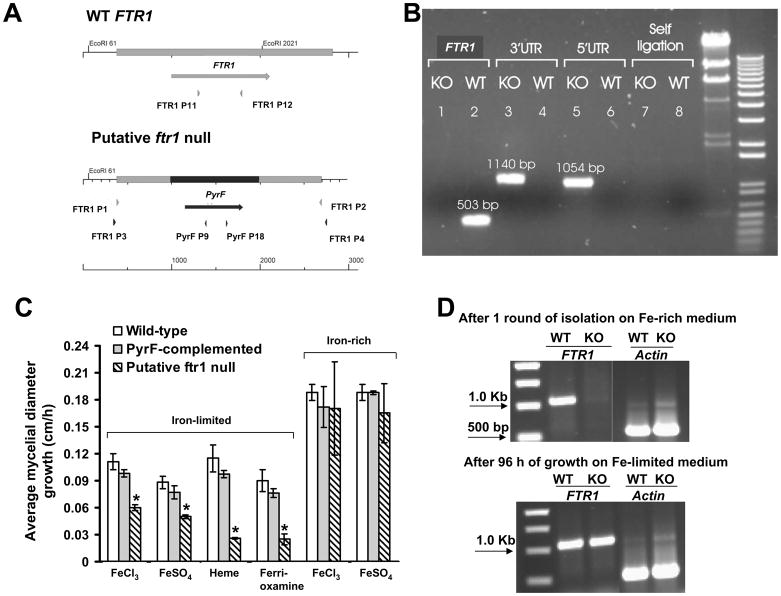
Disruption cassette integrates in FTR1 locus but complete elimination of FTR1 could not be achieved. (A) A diagram summarizing the strategy used to achieve FTR1 disruption. PyrF (998 bp) was used as a selectable marker flanked by 606 and 710 bp fragments of FTR1-5′ UTR and FTR1-3′ UTR, respectively. (B) Gel electrophoresis showing integration of the disruption cassette in a representative putative ftr1 null mutant (KO) but not in the wild-type (WT) (see 5′UTR and 3′UTR). Primers FTR1 P11 and FTR1 P12 were used to amplify 503 bp from the FTR1 ORF only from the wild-type but not from the putative ftr1 null mutant (see FTR1). Primers PyrF P9 and PyrF P18 to test for possible reciculization of the transformed plasmid with expected band of 2094 bp were also used (see self ligation). (C) Comparison of growth rate of R. oryzae wild-type, R. oryzae PyrF-complemented, or putative ftr1 null mutants grown on different sources of iron on iron-limited or iron-rich media. Growth was measured after 48 h for media containing 10 or 1000 μM of FeCl3 or FeSO4 or 100 μM of ferrioxamine, while growth was measured after 72 h for medium supplemented with 100 μM heme. Values (n=12 from four independent transformants with their growth measured in three experiments with similar results) are expressed as increase in mycelial diameter growth on solid growth medium in cm/h. * P<0.05 compared to wild-type or R. oryzae PyrF-complemented strains. (D) Gel electrophoresis showing lack of amplification of FTR1 after one round of purification of the putative null mutants on iron-rich medium (1000 μM FeCl3) and amplification of the FTR1 from the same isolate following growth on iron-depleted medium (i.e. 100μM ferrioxamine) for 96 h. Amplification of actin (600 bp) was used to control for DNA loading.