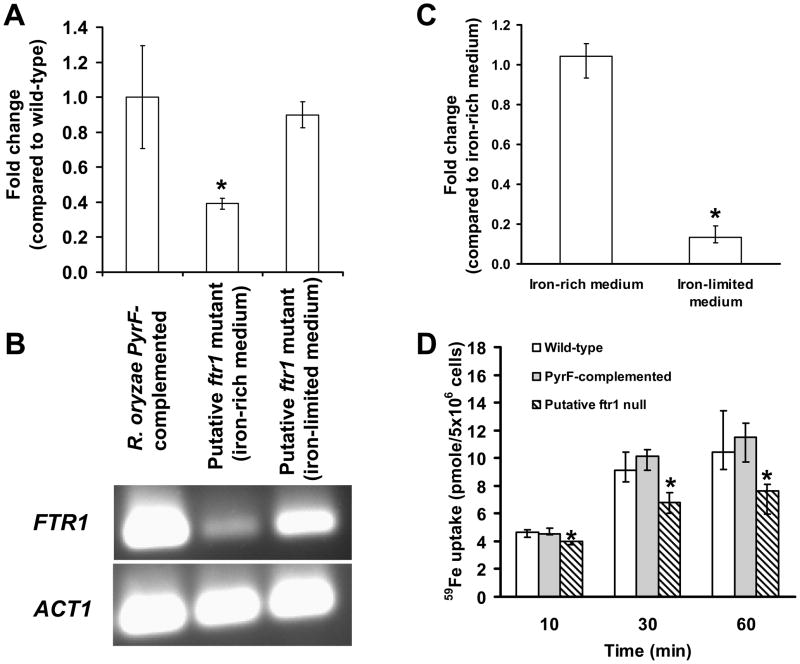
Fig. 4.
Reduced copy number results in compromised ability of R. oryzae to take up iron. (A) qPCR demonstrating reduced copy number of the wild-type FTR1 in the putative ftr1 null mutant compared to R. oryzae PyrF-complemented strain or to the same mutant grown in iron-depleted medium. (B) Gel electrophoresis of samples taken from the qPCR tube showing the amplification specificity for the FTR1 product. (C) qPCR demonstrating reduced copy number of the disrupted ftr1 allele in the putative ftr1 null when grown in iron-limited medium following passing in iron-rich medium (n=9 samples from two independent transformants). (D) The putative ftr1 mutant demonstrated reduced ability to acquire 59Fe compared to R. oryzae wild-type or R. oryzae PyrF-complemented strains. 59Fe uptake by wild-type, R. oryzae PyrF-complemented, or putative ftr1 mutant. Germinated spores were incubated with 0.1 μM 59FeCl3 (a concentration in which high-affinity iron permeases are induced (Fu et al., 2004)). *P <0.05 when compared with R. oryzae wild-type, R. oryzae PyrF-complemented strains, or the same strain grown on different concentrations of iron. Data (n= 9 from three separate experiments using three independent transformants) are expressed as medians + interquartile ranges.