Figure 3B.
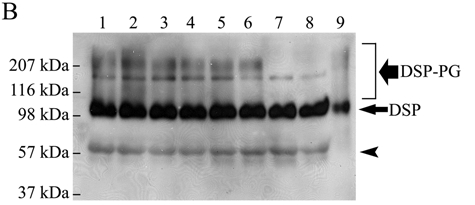
Substitutions of candidate Ser residues confirmed that 2 GAG chains are attached to the Ser242 and Ser254 in the mouse DSPP sequence. Lane 1, culture medium from HEK293-EBNA cells transfected with the pcDNA3-DSPP construct expressing normal mouse DSPP. Lane 2, from cells transfected with the construct expressing S201G-DSPP. Lane 3, from cells transfected with the construct expressing S242G-DSPP. Lane 4, from cells transfected with the construct expressing S254G-DSPP. Lane 5, from cells transfected with the construct expressing S201G+S242G-DSPP. Lane 6, from cells transfected with the construct expressing S201G+S254G-DSPP. Lane 7, from cells transfected with the construct expressing S242G+S254G-DSPP. Lane 8, from cells transfected with the construct expressing S201G+S242G+S254G-DSPP. Lane 9, 0.1 µg of pure DSP isolated from rat dentin (used as a positive control). The samples were treated with β-mercaptoethanol before being loaded onto the gels, and Western immunoblotting was carried out with the anti-DSP polyclonal antibody. The black smears migrating from ~ 100 kDa to > 207 kDa represent DSP-PG. A defined protein band migrating between the 116 and 207 kDa molecular-weight markers represents full-length mouse DSPP. Note that when both Ser242 and Ser254 were substituted (lanes 7 and 8), DSP-PG was completely lost; in contrast, if 1 of these 2 Ser residues was substituted, the amount of DSP-PG was reduced. The substitution of S201 did not reduce the quantity of DSP-PG. These findings confirmed that 2 GAG chains are attached to the Ser242 and Ser254 in the mouse DSPP sequence. Note that the amount of DSP (long/thin arrow) was very similar, but that of DSP-PG was different among lanes 1-8. The protein band (arrowhead) migrating above the 57-kDa molecular marker is likely to be a low-molecular-weight variant of mouse DSP (Goldberg et al., 2000; Qin et al., 2003).
