Fig. 3.
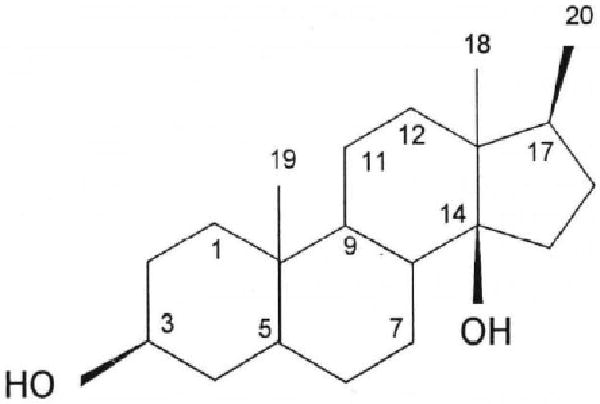
Prototypical cardenolide steroid skeleton. The primary feature is a steroid skeleton with the rings fused in a cis-trans-cis arrangement. The cardenolides discussed here have a 14βOH, an unsaturated lactone ring attached via C17 in the β configuration, and a methyl group at C18. When present, sugars are attached via the steroid 3βOH group. See Table 1 for the list of substituents in ouabain, ouabagenin, digoxin, digitoxin and Rostafuroxin. Reprinted with permission [99].
