Scheme 1.
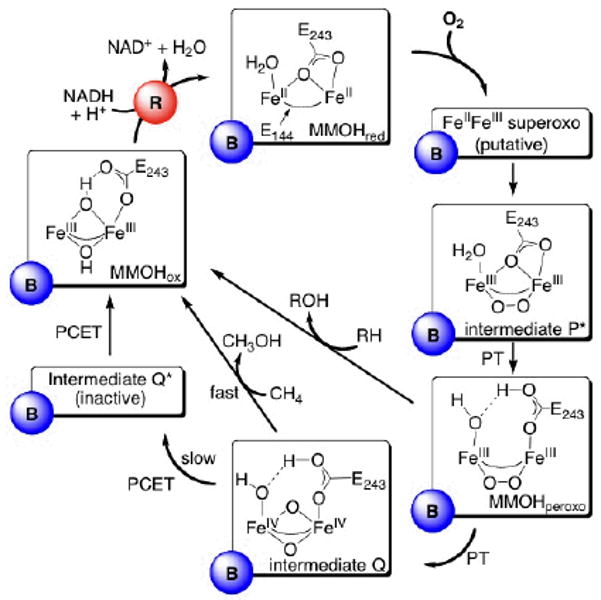
Catalytic cycle of O2 activation and CH4 hydroxylation in sMMO. The oxidized diiron(III) state (MMOHox) is activated via two-electron reduction by MMOR (R, red circle) to a diiron(II) state (MMOHred), which reacts in the presence of MMOB (B, blue circle) with dioxygen to form intermediate P*, presumably via a superoxo species. Intermediate P* then transforms via proton transfer (PT) into MMOHperoxo, which can either decay to MMOHox via oxidation of electrophilic substrates RH (e.g. ethers), or form the diiron(IV) intermediate Q, which is responsible for CH4 hydroxylation. In the absence of CH4, intermediate Q decays slowly to intermediate Q*, which is not on the methane activation pathway, and then to MMOHox. The bridging glutamates (E144 and E243) are also shown. Characteristic physical parameters of the intermediates can be found in the text.
