Abstract
Synthesis, in vitro and in vivo evaluation of [O-methyl-11C]dimethylamino-3(4-methoxyphenyl)-3H-pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-4-one (1), a potential imaging agent for mGluR1 receptors using PET are described. Synthesis of the corresponding desmethyl precursor 2 was achieved by demethylation of the methoxyphenyl compound 1 in 90% yield. Methylation using [11C]MeOTf in presence of NaOH afforded [11C]1 in 30% yield (EOS) with >99 % chemical and radiochemical purities and with a specific activity of 3–5 Ci/μmol (n = 6). The total synthesis time was 30 minutes from EOB. The radiotracer selectively labeled mGluR1 receptors in slide-mounted sections of postmortem human brain containing cerebellum, hippocampus, prefrontal cortex and striatum as demonstrated by in vitro autoradiography using phosphor imaging. PET studies in anesthetized baboon show that [11C]1 penetrates the BBB and accumulates in cerebellum, a region reported to have higher expression of mGluR1. These findings suggest [11C]1 is a promising PET radiotracer candidate for mGluR1.
Keywords: mGluR1 receptors, Autoradiography, Positron emission tomography, biological imaging, Human postmortem
Glutamate is the most abundant excitatory amino acid (EAA) in the mammalian brain and is involved in several pathological and physiological conditions (1). Metabotropic glutamate receptors (mGluRs) are G-protein coupled receptors (GPCR) involved in pain perception and are potential therapeutic targets for multiple diseases due to their ability to modulate excitatory glutamate transmission and postsynaptic signaling (2, 3). mGluRs are considered to be drug targets in order to modulate glutamate transmission in the treatment of various neurological and psychiatric diseases. Activation of group I receptors enhances the excitatory effects of glutamate by modulation of ion channel activity (4). Among the eight subtypes of mGluRs, mGluR1s are implicated in neuroprotection, pain, multiple sclerosis, motor dysfunction, epilepsy, cerebral ischemia and cerebellar long term depression (LTD) (5). Hence monitoring mGluR1, in vivo and non-invasively would lead to a better understanding of the role of this receptor in the pathophysiology of biological processes mediated through glutamergic pathways. However, in vivo quantification of the mGluR1 has been hindered by the lack of a successful PET tracer. Although the methyl analogue of the low affinity nonselective mGluR1 ligand CPCCO2Et, CPCCO2Me, is reported its pharmacological data as well as biological studies with [11C]CPCCO2Me have not been revealed (6). In a patent application, [3H]-radiolabeling of a variety of quinolines and quinolinones bearing mGluR1 antagonists were proposed for use in PET, but no data is available on their binding potential to mGluR1 (7). [11C]labeled mGluR1 antagonist, (3-ethyl-2-[11C]methyl-6-quinolinyl)(cis-4-methoxycyclohexyl)-methanone [11C]JNJ16567083), is a PET radioligand that was used for in vivo imaging in rats, however, no further data is available to support its effectiveness as an imaging agent, presumably due to low specific activity of the tracer (8). We selected dimethylamino-3(4-methoxyphenyl)-3H-pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-4-one 1) as a candidate PET ligand for mGluR1 due to its high affinity (Ki= 7.9 nM) and selectivity over mGluR5 (>1000 times) (9–11). Compound 1 was reported to be active in an in vivo model for pain (12). Moreover, presence of potent C-11 labeling site and a logP value 3.15, which is within the range for passive brain entry prompted us to develop [11C]1 as a PET ligand for mGluR1 (13).
Demethylation of compound 1, which is available through commercial vendors, using 1M BBr3 in DCM afforded the desmethyl precursor 2 in 90 % yield (14, Scheme 1). Methylation using [11C]MeOTf in presence of NaOH afforded [11C]1 in 30% radiochemical yield (EOS) with >99 % chemical and radiochemical purities (15). The specific activity was determined based on a five-point mass curve of the cold standard and typical specific activity ranged from 3 to 5 Ci/μmol (n = 6). Yield and specific activity of [11C]1 are sufficiently high to carry out routine imaging studies.
Scheme 1.
Synthesis of [11C]1.
Reagents and conditions: (a) BBr3, DCM, rt, 90%; (b) [11C]MeOTf, NaOH, rt, 30% (EOS).
After achieving the radiolabeling of ligand 1 in high yield and specific activity, [11C]1 was tested to obtain its binding to mGluR1 in human brain postmortem using phosphor image autoradiography (Figure 1). Frozen brain sections (20μm) of cerebellum, striatum, hippocampus and prefrontal cortex were used for the phosphor image study. Four sections each for total and nonspecific binding, per brain region, were assayed. Slide-mounted sections were brought to 22 °C and pre-incubated in 50 mM Tris-HCl, 1.2 Mm MgCl2, 2 mM CaCl2 and 0.1% BSA (pH 7.4) for 45 min at room temperature. This was followed by incubation in 12 nM of radioligand (SA = 1.88 Ci/micromole) for 60 minutes at room temperature. Adjacent sections were incubated with JNJ16259685 a known selective mGluR1 antagonist (10 μM) to determine nonspecific binding. After the incubation, sections were washed with ice-cold buffer containing 50 mM Tris- buffer (pH 7.4) at 4 °C and briefly dipped in ice-cold water to remove salts. Slides were quickly dried under a stream of cold air and exposed to a phosphor-imaging screen (Packard, ST screen wrapped in Mylar film) with high- and low-activity standards (American Radiolabeled Chemicals) for 90 minutes. Screens are scanned with a Packard Cyclone phosphor-imaging system and analyzed with OptiQuant Acquisition and Analysis software (Packard). Figure 1 illustrates specific binding of [11C]1 to mGluR1 and reveal specific binding and presence of high density of mGluR1in cerebellum (Bmax = 140 +/−20 fmol per sq cm), hippocampus (Bmax = 110 +/−25 fmol per sq cm), frontal cortex (Bmax 85 fmol per sq cm) and striatum (Bmax = 20 fmol per sq cm) human brain sections. High density of mGluR1 is adequate for quantifying the receptor by PET (16).
Figure 1.
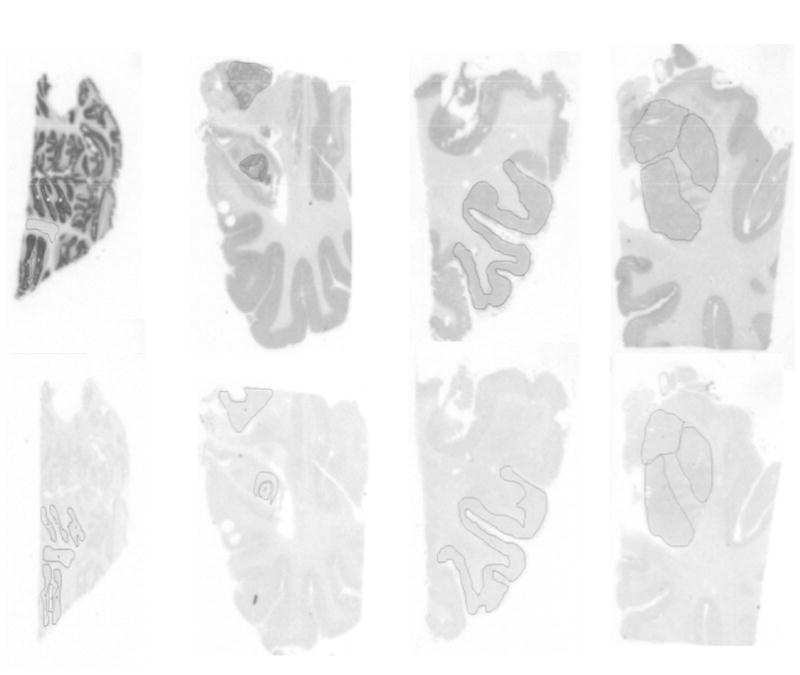
Phosphor images of [11C]1 in postmortem human brain sections.
Top row-Total and bottom row-nonspecific. First column: Cerebellum; second column: hippocampus; third column: frontal cortex; fourth column: striatum.
Subsequently, we performed PET studies of [11C]1 in anesthetized baboons (17). PET images shows that [11C]1 penetrates the BBB and accumulates in brain (Figure 2). Time activity curves (TACs) indicate rapid washout of radioligand in almost all brain regions except cerebellum where mGluR1 is abundant (Figure 3). The radioactivity levels of most of the regions apart from cerebellum reached baseline at 35 minutes. Ratio of most of the other brain regions to cerebellum was 2, 1.65 and 1.6 at 35, 65 and 85 minutes respectively. Metabolite analyses show 45% and 30% unmetabolized [11C]1 at 30 and 90 minutes. The radioligand did not exhibit considerable protein binding in human and baboon plasma. The reason for the faster washout of the tracer from other regions apart from cerebellum is not clear. However, the radioligand shows specific binding in in vitro phosphor image studies. A species difference may attribute to this discrepancy.
Figure 2.
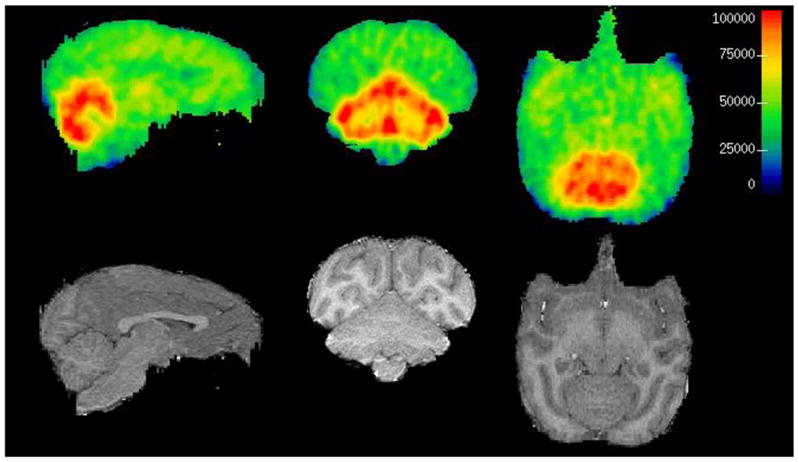
PET and MRI coregistered images of [11C]1 in baboon
First row: Baboon PET image; second row: MRI; First column: axial, middle column: coronal, last column: sagittal views.
Figure 3.
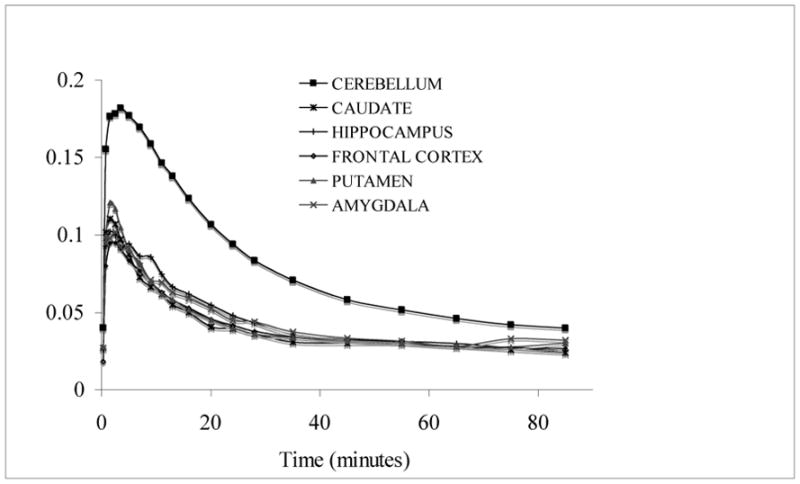
Time activity curves of [11C]1 in baboon
In summary, we successfully synthesized [11C]1, a potential imaging agent for mGluR1 receptors. The total time required for the radiosynthesis was 30 minutes from EOB using [11C]methyl triflate in acetone. [11C]1 was obtained in 30% yield (EOS) with >99 % chemical and radiochemical purities with a specific activity ranged from 3–5 Ci/μmol (n = 6). Phosphor image studies indicate that this newly developed [11C]1 ligand binds to mGluR1 enriched brain regions. PET studies in baboon show that [11C]1 penetrates BBB and is retained in cerebellum, a region with higher expression of mGluR1. Hence [11C]1 has the potential for the quantification of mGluR1 receptors in brain in vivo using PET. Further detailed in vitro and in vivo investigation of [11C]1 are in progress.
Acknowledgments
Financial support by the National Institute of Mental Health is gratefully acknowledged (R21 MH081801).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Hinoi E, Takarada T, Tsuchihashi Y, Yoneda Y. Curr Drug Targ: CNS Neurol Disord. 2005;4:211. doi: 10.2174/1568007053544093. [DOI] [PubMed] [Google Scholar]
- 2.Schoepp DD, Monn JAP. Glutamate and GABA Receptors and Transporters. 2002:151. [Google Scholar]
- 3.Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Nature Rev Drug Discov. 2005;4:131. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 4.Moghaddam BT. Psychopharmacol. 2004;174:39. [Google Scholar]
- 5.Gasparini F, Kuhn R, Pin J-P. Cur Opinion Pharmacol. 2002;2:43. doi: 10.1016/s1471-4892(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 6.Yu M, Brownell A-L. Molecular Imaging (Symposium) 2002:230. [Google Scholar]
- 7.Lesage ASJ, Bischoff FP, Janssen CGM, Lavreysen H. WO 2003082350 PCT Int Appl. 2003
- 8.Huang Y, Narendran R, Bischoff F, Guo N, Zhu Z, Bae S-A, Lesage AS, Laruelle MA. J Med Chem. 2005;48:5096. doi: 10.1021/jm050263+. [DOI] [PubMed] [Google Scholar]
- 9.Matasi JJ, Tulshian D, Burnett DA, Wu W-L, Korakas P, Silverman LS, Sasikumar TK, Qiang L, Domalski MS. US Pat. 2006;152:535. [Google Scholar]
- 10.Sasikumar TK, Li Q, Burnett DA, Greenlee William J, Li C, Heimark L, Pramanik B, Grilli M, Bertorelli R, Lozza G, Reggiani A. Bioorg Med Chem Lett. 2009;19(12):3199. doi: 10.1016/j.bmcl.2009.04.104. [DOI] [PubMed] [Google Scholar]
- 11.Wu W-L, Burnett DA, Domalski D, Greenlee WJ, Li C, Bertorelli R, Fredduzzi S, Lozza G, Veltri A, Reggiani AJ. Med Chem. 2007;50:5550. doi: 10.1021/jm070590c. [DOI] [PubMed] [Google Scholar]
- 12.Varty GB, Grill M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, Hodgson RA, Sherry XL, Nicolussi E, Pond AJ, Parker EM, Hunter JC, Higgins GA, Reggiani A, Bertorelli R. Psychopharmacol. 2005;179:207. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- 13.Wilson AA, Jin L, Garcia A, DaSilva JN, Houle S. Appl Rad Isotop. 2001;54(2):203. doi: 10.1016/s0969-8043(00)00269-4. [DOI] [PubMed] [Google Scholar]
- 14.O-methyl compound 1 (140 mg, 0.4 mmol) is dissolved in methylene chloride (5 mL) and BBr3 (1M, solution in methylene chloride, 0.6 mL, 0.44 mmol) was added to it. The solution was stirred for 1 h at room temperature. The reaction mixture was cooled in an ice-bath and quenched by adding methanol. The mixture was then poured into 5 mL NH4OH solution and extracted with methylene chloride. The combined organic extracts were dried over anhydrous MgSO4 and the solvent was removed under vacuum to provide the crude product. The product was chromatographed over silica gel using a mixture of 1:1 methylene chloride and ethyl acetate to obtain 120 mg (90%) of the desmethyl compound 2. 1HNMR (CDCl3, 400 MHz) d ppm 8.40 (d, 1H), 7.58 (s, 1H), 7.10 (m, 2H), 6.85 (m, 3H), 2.47 (s, 6H) HRMS calculated for C17H15N4O2S; 338.0840: Found; 338.0838.
- 15.Radiosynthesis of [11C]1: The precursor desmethyl-1 (0.5 to 1.0 mg) was dissolved in 500 μL of acetone in a capped 5 mL V-vial. Sodium hydroxide (10 μL, 5 M) was added and the resultant solution was allowed to stand for 2 minutes. High specific active [11C]CO2 produced from RDS112 Cyclotron (~37 GBq) was subsequently converted into [11C]CH3OTf and is transported by a stream of argon (20–30 mL/min) into the vial over approximately 5 minutes at room temperature. At the end of the trapping, the product mixture was diluted with 0.5 ml of acetonitrile and is directly injected into a semi preparative RP-HPLC (Phenomenex C18, 10 × 250 mm, 10) and eluted with acetonitrile: 0.1 M ammonium formate solution (40:60) at a flow rate of 10 mL/min. The precursor eluted after 3–4 minute during the HPLC analysis. The product fraction with a retention time of 7–8 minutes based on γdetector was collected, diluted with 100 mL of deionized water, and passed through a classic C-18 Sep-Pak® cartridge. Reconstitution of the product in 1 mL of absolute ethanol afforded [11C]1 (30% yield, based on [11C]CH3I at EOS). A portion of the ethanol solution was analyzed by analytical RP-HPLC (Phenomenex, Prodigy ODS(3) 4.6 × 250 mm, 5; mobile phase: acetonitrile/0.1 M ammonium formate; 45: 55, flow rate: 2 mL/min, retention time: 8 min, wavelength: 230 nm) to determine the specific activity and radiochemical purity. Then the final solution of the [11C]1 in 10% ethanol-saline was analyzed to confirm the chemical and radiochemical purities.
- 16.The autoradiograms were quantified using a dilution series (each 1:10) of droplets (5uL) made from the incubation solution, these were added to a TLC plate and exposed together with the autoradiograms. The pixel intensity of the droplets were fitted by a power equation using counted samples as concentration references. Individual ROI’s were thereby quantified for the total amount of compound using the specific activity.
- 17.Baboon PET scanning with [11C]1: Fasted animals were immobilized with ketamine (10 mg/kg, i.m.), and anesthetized with 1.5~2.0 % isoflurane via an endotracheal tube. Core temperature was kept constant at 37°C with a heated water blanket. An i.v. infusion line with 0.9% NaCl was maintained during the experiment and used for hydration and radiotracer injection. An arterial line is placed for obtaining arterial samples for the input function. After a 10 minute transmission scan, 5 ± 0.5 mCi of [11C]1 (S.A. of 2.5 ± 0.5 Ci/μmol) was injected as an i.v. bolus and emission data were collected for 120 minutes in 3-D mode in a Siemens ECAT EXACT HR+ (CPS/Knoxville, TN). The head was positioned at the center of the field of view as defined by imbedded laser lines. Regions of interest drawn on the animal’s MRI scan were transferred to co-registered (AIR) frames of PET data.


