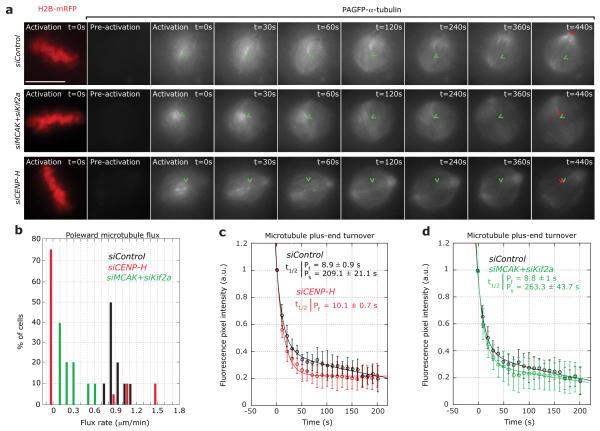
Figure 2.
Loss of CENP-H abrogates MT flux and abolishes control of kMT turnover (a) Successive frames every 30 seconds before and after photoactivation of stable PAGFP-α-tubulin/H2B-mRFP HeLa cells treated with siControl, siCENP-H or siMCAK+siKif2a RNAs. PAGFP-α-tubulin fluorescence was activated in a circular region near the chromosome mass in metaphase cells (detected by the H2B-mRFP signal). A H2B-mRFP (DNA) frame is shown for the first time point of the live-cell movie after activation. (b) Quantification of poleward MT flux rates in cells treated with siControl (black bars), siCENP-H (red bars) or siMCAK+siKif2a (green bars) RNAs. n = 20 cells each (c-e) Quantification of fluorescence intensity decay of the activated regions over time in siControl (black), siCENP-H (red) or siMCAK+siKif2a (green) treated cells. The lines through the data points were fitted to a double exponential equation of the type I = Pf.exp(−kf.t) + Ps.exp(−ks.t), which correspond to previously described slow and fast MT populations7, 19. Analysis of the siCENP-H fluorescence loss indicated that the data fitted to single exponential curve (R2 = 0.99). Indicated are the corresponding half-lives of the fast and slow MT populations. Green arrowheads mark the initial position of the photoactivated spot and the red arrowheads show the final position of the activated spot. Scale bar = 10 μm. Error bars represent SD.