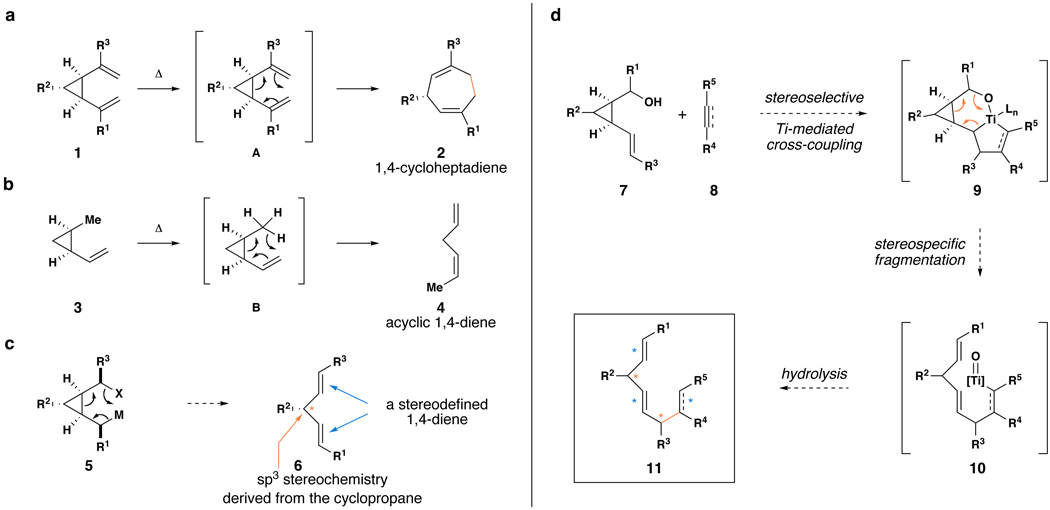
Figure 3. The design of a convergent coupling reaction suitable for the stereoselective synthesis of complex skipped polyenes.
a, Cope rearrangement of cis-divinylcyclopropanes. b, 1,5-hydrogen migrations in cis-vinylcyclopropanes. c, A plausible 6-electron process for acyclic 1,4-diene synthesis – alkene geometry is set as a function of the stereochemistry of the intermediate and the mechanistic course of the fragmentation. d, Design of a cross-coupling/fragmentation cascade for preparation of stereodefined skipped polyenes.  = stereochemistry of up to three alkenes is established;
= stereochemistry of up to three alkenes is established;  = this carbon–carbon bond forming process has the potential to establish 1,4-dienes bearing a central stereodefined C3-carbon;
= this carbon–carbon bond forming process has the potential to establish 1,4-dienes bearing a central stereodefined C3-carbon;  = carbon–carbon bond formed in Ti-mediated cross-coupling; M = metal.
= carbon–carbon bond formed in Ti-mediated cross-coupling; M = metal.