Table 1.
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | allylic alcohol or diazoacetate starting material |
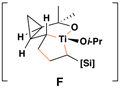
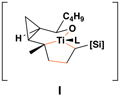
vinylcyclopropane | Presumed metallacyclic intermediatea |
Yield (%)b |
(E:Z)c | drc | productd |
| 1 | 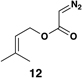 |
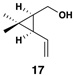 |
 |
58 (82% brsm) |
20:1 | – | 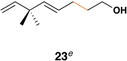 |
| 2 | 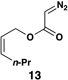 |
 |
 |
54 | 20:1 | – | 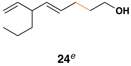 |
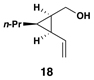
| 3 | 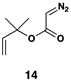 |
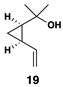 |
 |
50 | 20:1 | – | 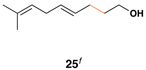 |
| 4 | 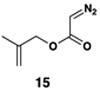 |
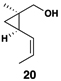 |
 |
61 | 10:1 | – | 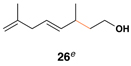 |
| 5 | 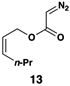 |
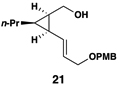 |
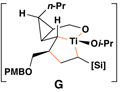 |
55 | 20:1 | 20:1 | 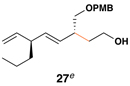 |
| 6 | 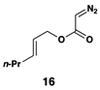 |
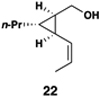 |
 |
52 | 20:1 | 20:1 | 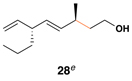 |
| 7 | 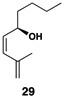 |
 |
 |
52 | 20:1 | 20:1 |  |
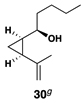
| 8 | 29 | 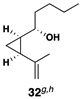 |
 |
42 | 20:1 | 20:1 | 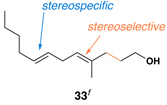 |
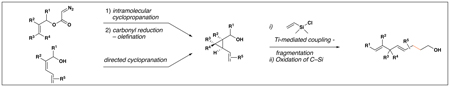
 = bonds formed in the coupling process.
= bonds formed in the coupling process.
While not computed for examples 2–8, as seen in entry 1, these reactions did not consume all of the vinylcyclopropane starting material.
No evidence was found for the production of stereoisomeric products.
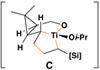
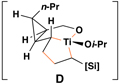
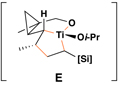
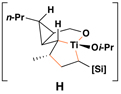
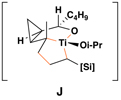
Reaction conditions for cross-coupling: vinylsilane, ClTi(Oi-Pr)3, c-C5H9MgCl, Et2O (−78 to −50 °C), then add Li-alkoxide of vinylcyclopropane (−70 °C to rt over 3 h).
Oxidation conditions: TBHP, H2O, CsOH•H2O, TBAF, DMF, 70 °C.
Oxidation conditions: KF, KHCO3, H2O2, MeOH, THF.
ICH2Cl, Sm[Hg], THF, (85%, dr ≥ 20:1).
PDC, 4 Å sieves, CH2Cl2 (91%), then L-Selectride, THF (76% of desired isomer, dr = 6:1).
