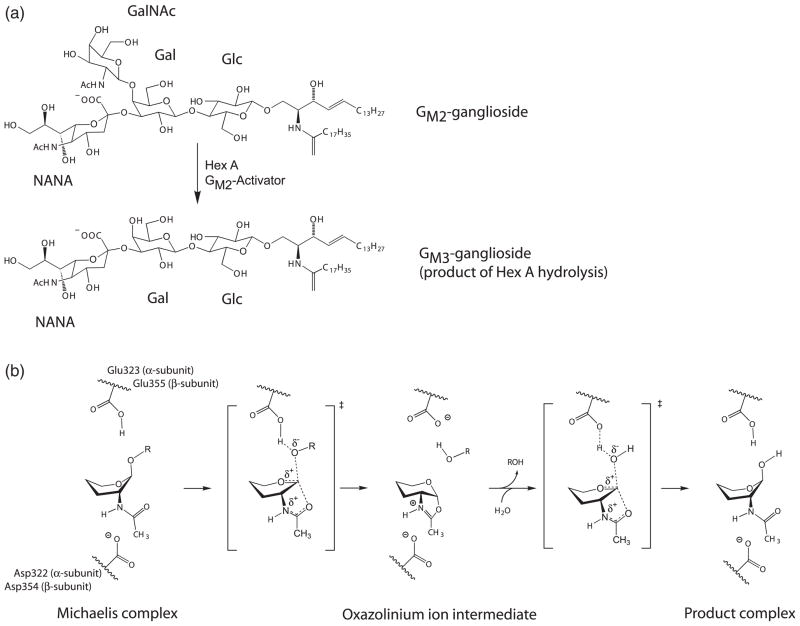
Figure 3.
Proposed catalytic mechanism for Hex A. (a) Hydrolysis of the GM2 ganglioside by Hex A results in the loss of GalNAc to produce a GM3 ganglioside. (b) Proposed catalytic mechanism for Hex A showing substrate-assisted catalysis. αGlu323 in the α-subunit and βGlu355 in the β-subunit act as the general base, while αAsp322 in the α-subunit and βAsp354 in the β-subunit act to orient the C2-acetamido group into position for nucleophilic attack and subsequently stabilizes the oxazolinium ion intermediate. The hydroxyl residues and C6 have been removed from the pyranose ring of the substrate for clarity. The exact positions for these groups have not been determined.