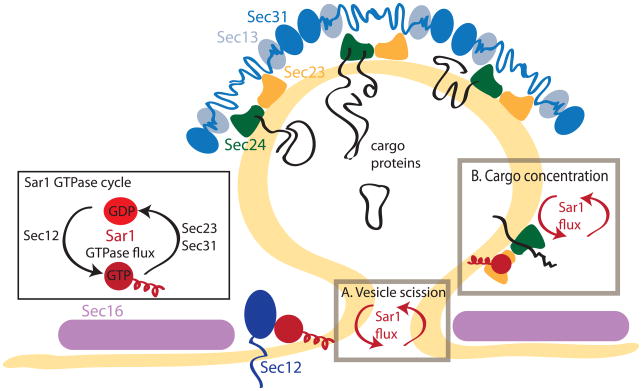
Fig. 1. COPII assembly and the Sar1 GTPase cycle.
The COPII coat assembles on the ER membrane through the coordinated action of a number of components. The small G-protein, Sar1, is recruited to the ER membrane by its guanine nucleotide exchange factor (GEF), Sec12, exposing an amphipathic α-helix upon GTP binding. Sar1•GTP in turn recruits the Sec23/Sec24 dimer and the Sec13/Sec31 tetramer to create the coat polymer. The GTPase cycle of Sar1 (inset) is controlled by the GEF, Sec12, and the GTPase activating protein (GAP), Sec23. Sec31 further stimulates the GAP activity of Sec23. Sec16 stably marks ER exit sites and is required for their integrity. Recent evidence suggests that instead of a rigid stepwise assembly of the coat, flux of Sar1 through the GTP cycle is required for specific events in the biogenesis of a COPII vesicle. A. Vesicle release from the donor membrane is impaired when the N-terminal helix of Sar1 is removed [12] or when GTP hydrolysis is prevented [13]. B. Concentration of cargo proteins in a minimally reconstituted system is reduced when Sar1 cannot hydrolyse GTP [19••]. Repeated Sar1 GTPase cycles could promote higher affinity associations between Sec23/24 and ER export signals on cargo.