Summary
Cellular aging is characterised by telomere shortening, which can lead to uncapping of chromosome ends (telomere dysfunction) and that activation of DNA damage responses. There is some evidence the DNA damage accumulates during human aging and that lifestyle factors contribute to the accumulation of DNA damage. Recent studies have identified a set of serum markers that are induced by telomere dysfunction and DNA damage and these markers showed an increased expression in blood during human aging. Here, we investigated the influence of lifestyle factors (such as exercise, smoking, body mass) on the aging associated expression of serum markers of DNA damage (CRAMP, EF-1α, Stathmin, n-acetyl-glucosaminidase, and chitinase) in comparison to other described markers of cellular aging (p16INK4a upregulation and telomere shortening) in human peripheral blood. The study shows that lifestyle factors have an age-independent impact on the expression level of biomarkers of DNA damage. Smoking and increased body mass indices were associated with elevated levels of biomarkers of DNA damage independent of the age of the individuals. In contrast, exercise was associated with an age-independent reduction in the expression of biomarkers of DNA damage in human blood. The expression of biomarkers of DNA damage correlated positively with p16INK4a expression and negatively with telomere length in peripheral blood T-lymphocytes. Together, these data provide experimental evidence that both aging and lifestyle impact on the accumulation of DNA damage during human aging.
Introduction
There is an ongoing effort to identify biomarkers of human aging. Biomarkers of aging could be used in clinical settings to predict disease outcomes and sensitivity of elderly patients to invasive therapies. In addition, there are growing efforts to develop molecular therapies and lifestyle interventions that slow down the aging process. However, the evaluation of the efficacy of such approaches is extremely difficult since the measurement of effects on fitness and lifespan would require long lasting clinical studies. The development of molecular markers that not only indicate the biological age of an individual but are also sensitive to intervention and lifestyle factors would represent an important step for the future development of anti-aging therapies/interventions.
There is growing evidence that an accumulation of nuclear DNA damage can contribute to the impairments in maintenance and function of organs during aging and chronic disease (for review see Nalapareddy et al. 2008). In support of this hypothesis, it has been shown that mutations in DNA repair and checkpoint genes are the cause of some progeroid syndromes in humans (Lieber & Karanjawala 2004). A variety of studies have revealed evidence for an increase in DNA damage in aging human tissues. Specifically, γH2AX-positive DNA damage foci increase in aging lymphocytes (Sedelnikova et al. 2008), double strand breaks increase in sperm of aged humans (Singh et al. 2003; Schmid et al. 2007), single strand DNA breaks increase in aging lymphocytes (Chicca et al. 1996) and muscle (Zahn et al. 1987), oxidative DNA lesions (7-methylguanine and 8-hydroxydeoxyguanosine) show an age-dependent increase in human urine (Tamae et al. 2009), and an age-dependent increase in cytogenetic abnormalities occurs in human lymphocytes (Ramsey et al. 1995).
Telomere shortening represents a cell intrinsic mechanism that can contribute to the accumulation of DNA damage during aging. Telomeres cap the chromosomal ends to preserve DNA integrity and chromosomal stability. Telomeres shorten 50–100 base pairs during each cell division due to the end-replication problem of the DNA polymerase and due to processing of telomeres during S-phase (Levy et al. 1992, Verdun and Karlseder 2007). Telomere shortening eventually leads to telomere dysfunction (chromosome uncapping) and the induction of DNA damage checkpoints resulting in a permanent cell cycle arrest and replicative senescence (d'Adda di Fagagna et al. 2003; Takai et al. 2003). p16 and p21 represent two cell cycle inhibitors that are up-regulated in senescent cells (Alcorta et al. 1996, Stein et al. 1999). Functional studies on senescent fibroblasts have shown that p21 is a downstream target of p53 and telomere dysfunction, whereas p16 appears to be upregulated in a p53 and telomere independent manner (Herbig et al. 2004).
Both telomere shortening and the up-regulation of p16 have been associated with aging. Telomere shortening occurs in the vast majority of human tissues during aging and it is conceivable that this process contributes to the accumulation of DNA damage in aging human tissues (for review see Jiang et al. 2007). Correlative studies in humans have found that short-for-age telomere length is associated with decreased longevity and an increased development of certain age-associated diseases, particularly cancer, cardio-vascular disease, and deleterious infection (see for example Cawthon et al. 2003; Farzaneh-Far et al. 2008; Yang et al. 2009). An upregulation of p16 has been observed in various tissues of aging mice as well as in humans (Ressler et al. 2006, Krishnarmurthy et al. 2004).
Studies on telomerase knockout mice (Terc−/−) have provided the first experimental evidence that telomere dysfunction can impair the maintenance of organ systems leading to premature aging (Rudolph et al. 1999; Choudhury et al. 2007; Schaetzlein et al. 2007). Mutations of genes that are required for telomerase activity also lead to accelerated telomere shortening, organ failure and a shortened lifespan in humans, for example in patients with dyskeratosis congenita (for review see Gu et al. 2009; Walne & Dokal 2009). These findings suggest that human telomeres have a limited length and telomere shortening can represent a causal factor influencing human aging, the evolution of age associated diseases and lifespan.
There is evidence that telomere shortening and the accumulation of DNA damage is influenced by lifestyle factors such as smoking, obesity and exercise. Specifically, it has been shown that smoking increases oxidative DNA damage, whereas exercise and physical activity reduces it (Tamae et al. 2009). In contrast, it has also been observed that excessive exercise (such as half marathon running) can lead to transient increases in single stranded DNA breaks in peripheral blood cells (Niess et al. 1998). The age-dependent accumulation of cytogenetic abnormalities in lymphocytes was accelerated by smoking (Ramsey et al. 1995). Studies on peripheral blood lymphocytes have suggested that lifestyle factors associate with telomere length (Valdes et al. 2005; Cherkas et al. 2006; Epel et al. 2006; Cherkas et al. 2008; Epel et al. 2009; Mirabello et al. 2009) and the accumulation of oxidative DNA damage, single strand DNA breaks and DNA adducts (Thomson et al. 2005; Hofer et al. 2006; Mizoue et al. 2007; Rundle et al. 2007, Allgayer et al. 2008; Hofer et al. 2008; O'Callaghan et al. 2009).
Together, there is growing evidence that age and lifestyle dependent rates of telomere shortening and DNA damage accumulation could contribute to human aging and disease. However, the measurement of telomere length and DNA damage has not yet been implemented in clinical settings, except from clinical testing of bone marrow failure and leukemia in young adults. This is partly due to the weak correlation of telomere length with age and the complexity of the assays for telomere length measurement. We have recently identified a set of biomarkers (CRAMP, Stathmin, EF-1α, and chitinase enzyme activity) that are induced by telomere dysfunction or γ-irradiation (Jiang et al. 2008). Our previous study indicated that the expression of these proteins is induced by DNA damage, although the exact level of DNA damage or the exact number of dysfunctional telomeres per cell that induce the expression of these marker proteins is currently unknown. The functional role of an increased expression of these marker proteins in response to DNA damage or telomere dysfunction also remains to be investigated. However, these proteins could represent useful biomarkers since the expression of these proteins can easily be measured in human blood serum using ELISA and enzyme activity assays. The expression of these marker proteins was found to increase in human blood serum in association with aging and the occurrence of age-associated diseases (Jiang et al. 2008).
Here, we analyzed the influence of lifestyle factors on the expression of these biomarkers in blood serum and we determined its correlation to well-described markers of cellular senescence (upregulation of p16INK4a, telomere shortening) in blood cells of the same individuals. The study shows that lifestyle factors (specifically smoking, body mass index, and exercise) have an age-independent impact on the expression of serum markers of telomere dysfunction and DNA damage and the expression of these markers correlates with biomarkers of cellular senescence in peripheral blood T-lymphocytes. These findings suggest that biomarkers of DNA damage and telomere dysfunction could help to evaluate the efficacy of lifestyle interventions and future therapies aiming to slow down the aging process.
Results
Age correlates with expression level of serum markers of DNA damage and telomere dysfunction
In previous studies we have identified a set of serum markers that showed an increased expression in culture medium of telomere dysfunctional cells and γ-irradiated cells compared to culture medium from young, non-irradiated cells (Jiang et al. 2008). Moreover, the expression of these serum markers showed an increased expression during human aging and age-associated diseases (Jiang et al. 2008). Here, the expression of these biomarkers was measured by ELISA (for CRAMP, EF-1α and Stathmin) and enzyme activity assays (for n-acetyl-glucosaminidase, chitobiosidase, and chitotriosidase) in serum samples of a previously described cohort of 170 healthy persons aged from 18–80 years (Liu et al. 2009). For the current study a set of 136 serum samples was available from the previous described cohort. The best combination score of serum biomarkers was calculated according a population-based stochastic search method (particle swarm optimization algorithm), which was used to maximise the rank correlation of a linear combination of all marker subsets under the constraint of positive weight (for more details see supplementary material, pages 1–3). In addition, the data were correlated to telomere length and the previously analyzed expression level of p16INK4amRNA in peripheral blood T-lymphocytes (PBTLs) of the same cohort (Liu et al. 2009).
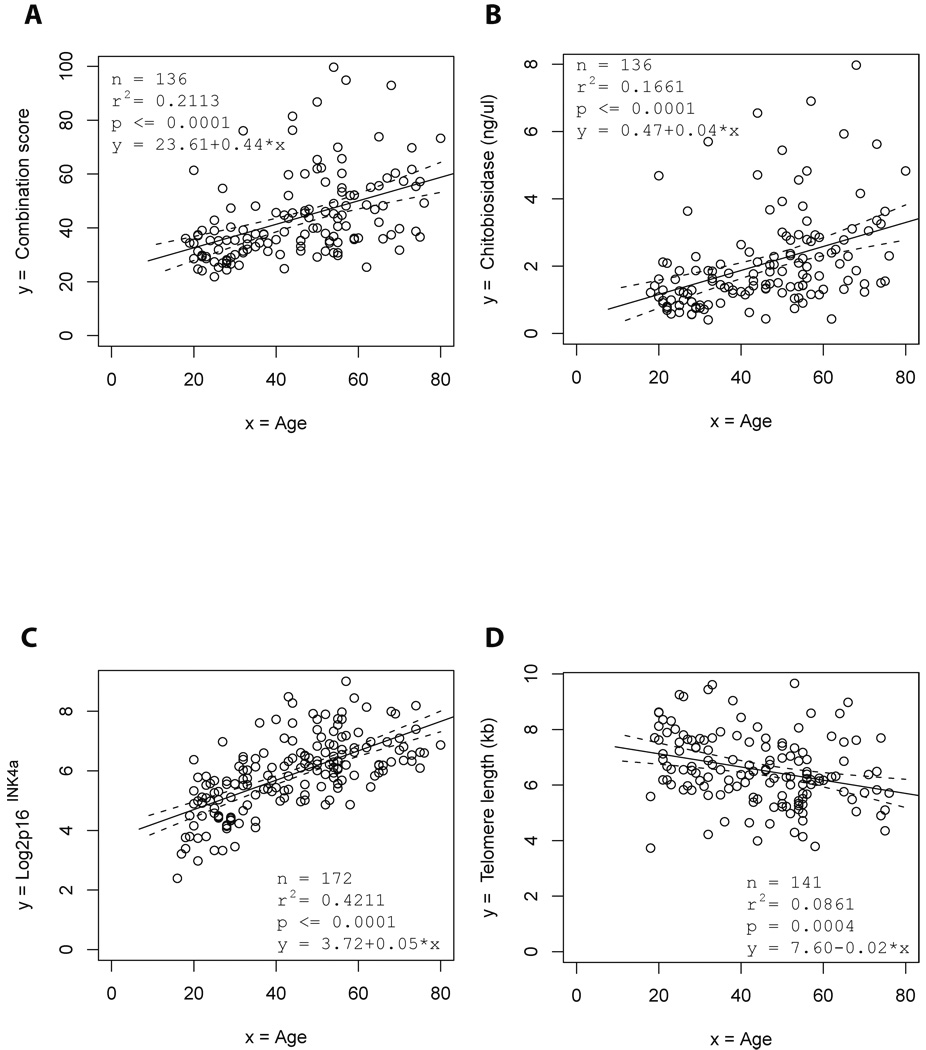
In agreement with our previous studies, the expression of the serum markers (except CRAMP) showed a significant age-dependent increase in the current cohort (data not shown). The best calculated combination score correlating to age of individuals in the current cohort was based on the expression of Stathmin, n-acetyl-glucosaminidase, and chitobiosidase (Fig. 1A and supplementary material, page 3). In addition to the previously identified markers, the enzyme activity of chitobiosidase and chitotriosidase (both parameters measure chitinase activity) showed a significant age-dependent increase in chitinase activity in blood serum (Fig. 1B, and data not shown for chitotriosidase). Consistent with previous studies, analysis of peripheral blood T-lymphocytes (PBTLs) revealed that expression of p16INK4a mRNA was positively correlated with age and telomere length was negatively correlated with age (Fig. 1C, D). Together, these data confirmed previous results showing an age-dependent increase in marker proteins of DNA damage and telomere dysfunction in human blood serum.
Figure 1. The expression of serum markers of telomere dysfunction and DNA damage is directly associated with chronological age.
The graphs show the association of the indicated biomarkers with age of the blood donors. Regression analysis shows the association of age with (A) the best combination of serum levels of biomarkers of telomere dysfunction and DNA damage (combination score equals to 0.1168 × stathmin + 0.0679 × n-acetyl-glucosaminidase + 8.8630 × chitobiosidase), (B) the level of chitobiosidase activity in blood serum, (C) the expression of P16 INK4a mRNA in PBTLs, (D) the telomere length in PBTLs. The black line shows the regression line and the dashed lines the 95% confidence bands for the regression line.
Lifestyle factors impact on the expression level of serum markers of DNA damage and telomere dysfunction
To evaluate the role of lifestyle factors on the accumulation of DNA damage markers in human blood, a questionnaire was used evaluating tobacco smoking and physical exercise. In addition, the body mass index of all individuals was determined at the time of blood withdrawal.
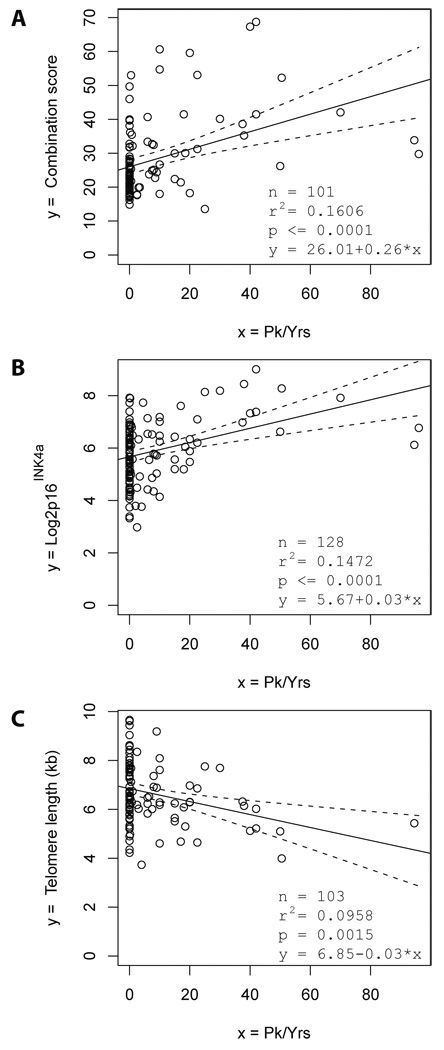
We calculated a combination score of biomarkers of DNA damage and telomere dysfunction, which showed the best correlation to smoking (measured in pack years, n=103 individuals). The best combination score was based on the expression of stathmin and chitobiosidase (supplementary material, page 3). The combination score indicated a direct association between the dosage of smoking and the accumulation of DNA damage and telomere dysfunction (Fig. 2A). Of note, regression analysis including age as a confounding factor revealed an age-independent, direct association between the dosage of smoking and DNA damage accumulation (Table 1A, p=0.002). The partial correlation between the dosage of smoking (pack-years) and the combination of serum markers after correction for age was 0.30179.
Figure 2. The dosage of smoking is directly associated with the expression of serum markers of telomere dysfunction and DNA damage.
(A–C) The Regression analysis shows the correlation of tobacco smoking (measured in pack years: 1 pack per day for 1 year = 1 pack year) with (A) the best combination of serum markers of telomere dysfunction and DNA damage (combination score equals to 0.0942 × stathmin +7.2519 × chitobiosidase), (B) with the expression of P16INK4a mRNA in PBTLs, and (C) with telomere length in PBTLs. The black line shows the regression line and the dashed lines the 95% confidence bands for the regression line.
Table 1.
Summary statistics for fitted regression models
| A) | |||||
|---|---|---|---|---|---|
| Comb Pk/Yrs: n = 103 | |||||
| Variable | Coefficient (β) |
Standard Error |
95% CI | t Stat | P |
| Intercept | 17.152 | 2.966 | 11.268 to 23.036 |
5.783 | <10−5 |
| Age | 0.2188 | 0.068 | 0.084 to0.353 | 3.231 | 0.00167 |
| Pk/Yrs | 0.1903 | 0.060 | 0.071 to 0.309 | 3.165 | 0.00205 |
| B) | |||||
|---|---|---|---|---|---|
| Comb Exercise minutes per month: n = 80 | |||||
| Variable | Coefficient (β) |
Standard Error |
95% CI | t Stat | P |
| Intercept | 3041.330 | 296.967 | 2449.994 to 3632.665 |
10.241 | <10−5 |
| Age | 13.513 | 5.743 | 2.078 to 24.947 | 2.353 | 0.0212 |
| Exercise | −0.669 | 0.262 | −1.192 to −0.147 | −2.552 | 0.0127 |
| C) | |||||
|---|---|---|---|---|---|
| Comb BMI n=91 | |||||
| Variable | Coefficient (β) |
Standard Error |
95% CI | t Stat | P |
| Intercept | 3.013 | 0.395 | 2.227 to 3.799 | 7.618 | <10−5 |
| Age | 0.013 | 0.005 | 0.003 to 0.023 | 2.584 | 0.0114 |
| BMI | 0.036 | 0.013 | 0.010 to 0.061 | 2.797 | 0.0063 |
Residual standard error is 10.34 on 100 degrees of freedom.
Model R2 = 24%
Residual standard error is 782.9 on 77 degrees of freedom.
Model R2 = 19%
Residual standard error is 0.7743 on 88 degrees of freedom.
Model R2 = 16%
The dosage of tobacco smoking also showed a significant, direct association with the expression of p16INK4a mRNA, and an inverse association with telomere length (Fig. 2B, C). However, this associations were weaker compared to the association between smoking dosage and the combination score of the blood biomarkers of DNA damage and telomere dysfunction (Fig. 2A).
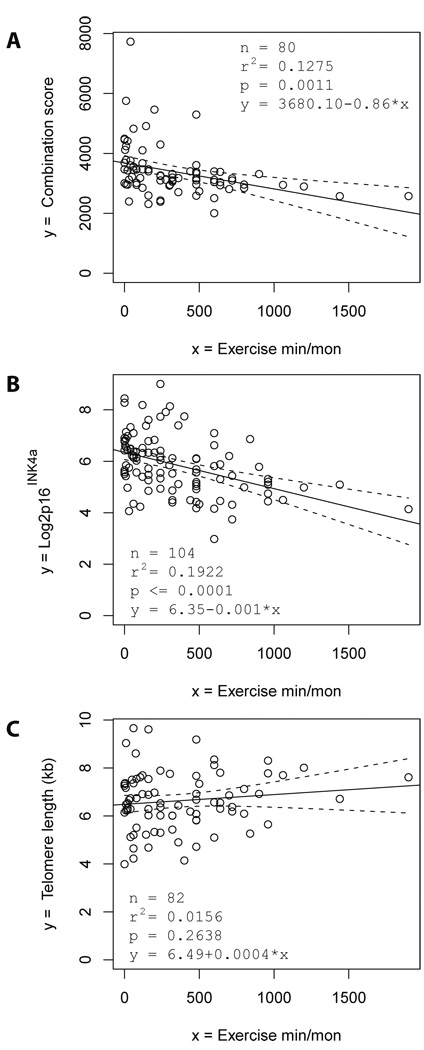
To evaluate an association of physical exercise with the expression of biomarkers of DNA damage and telomere dysfunction, exercise duration (minutes per month) was analysed in correlation to the expression of serum markers (n=80 individuals). The best biomarker combination score correlating to physical exercise was based on the expression of Stathmin, n-acetyl-glucosaminidase, chitotrioiosidase and CRAMP (supplementary material, page 3). This score indicated an inverse association between exercise duration and the accumulation of DNA damage and telomere dysfunction (Fig. 3A). Regression analysis including age as a confounding factor revealed an age-independent, inverse association between exercise duration and the accumulation of DNA damage (Table 1B, p=0.0127). The partial correlation between exercise duration (minutes per month) and the expression of biomarkers of DNA damage after correction for age was −0.277. In addition, physical exercise duration showed an inverse association with the level of p16INK4a mRNA expression (Fig. 3B), while telomere length was not significantly correlated with exercise (Fig. 3C). Together, the data suggested that exercise may exert protective effects impairing the age-dependent accumulation of cells with increased levels of DNA damage, telomere dysfunction or p16INK4a mRNA expression.
Figure 3. The duration of physical exercise is inversely associated with the expression of serum markers of telomere dysfunction and DNA damage.
(A–C) The regression analysis shows the correlation between physical exercise (measured in minutes per month) with (A) the best combination score of serum markers of telomere dysfunction and DNA damage (which equals to 11.6553 × stathmin + 9.7161 × n-acetyl-glucosaminidase + 0.0201 × chitotriosidase + 8.494 × CRAMP), (B) the expression of P16 INK4a mRNA in PBTLs, and (C) the telomere length in PBTLs. The black line shows the regression line and the dashed lines the 95% confidence bands for the regression line.
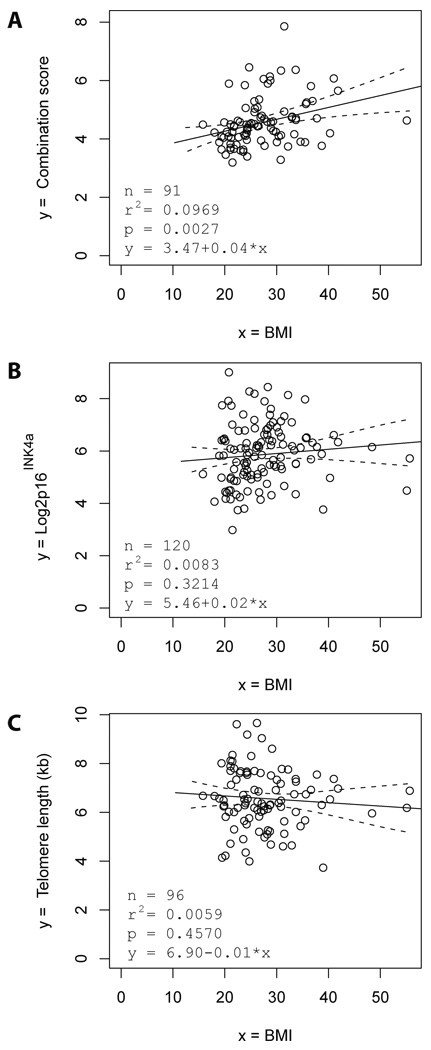
To evaluate an association of body weight with the expression of serum markers, the body mass indices (BMI) of the individuals (n=91) were evaluated at the time point of phlebotomy. The best biomarker combination score correlating to BMI was based on the expression of CRAMP, EF-1α, and chitobiosidase (supplementary material, page 3). This score showed a significant, direct association between the BMI and the accumulation of DNA damage and telomere dysfunction (Fig. 4A). Regression analysis including age as a confounding factor revealed an age-independent, direct association of the BMI with the accumulation of DNA damage (Table 1C, p=0.0063). The partial correlation between BMI and the combination of biomarkers of DNA damage after correction for age was 0.2856. Other parameters (p16INK4a mRNA expression and telomere length) were not significantly correlated to the BMI of the individuals (Fig. 4B,C).
Figure 4. The body mass index (BMI) is directly associated with the expression of serum markers of telomere dysfunction and DNA damage.
(A–C) Regression analysis shows the correlation of the body mass index (BMI) of the blood donors with (A) the best combination score of serum markers of telomere dysfunction and DNA damage (which equals to 0.0313 × chitobiosidase + 0.0097 × CRAMP + 4.151 × EF-1α), (B) the expression of P16 INK4a mRNA in PBTLs, and (C) the telomere length in PBTLs. The black line shows the regression line and the dashed lines the 95% confidence bands for the regression line.
Together, these data show that lifestyle factors (smoking, exercise, and body weight) have a significant, age-independent effect on the expression level of some biomarkers of telomere dysfunction and DNA damage in human blood.
Blood serum markers of DNA damage and telomere dysfunction correlate with senescence markers in peripheral blood T-lymphocytes
The expression of serum markers of DNA damage and telomere dysfunction was correlated to the mRNA level of p16INK4a as well as to the telomere length in peripheral blood T-lymphcoytes (PBTLs) from the same individuals.
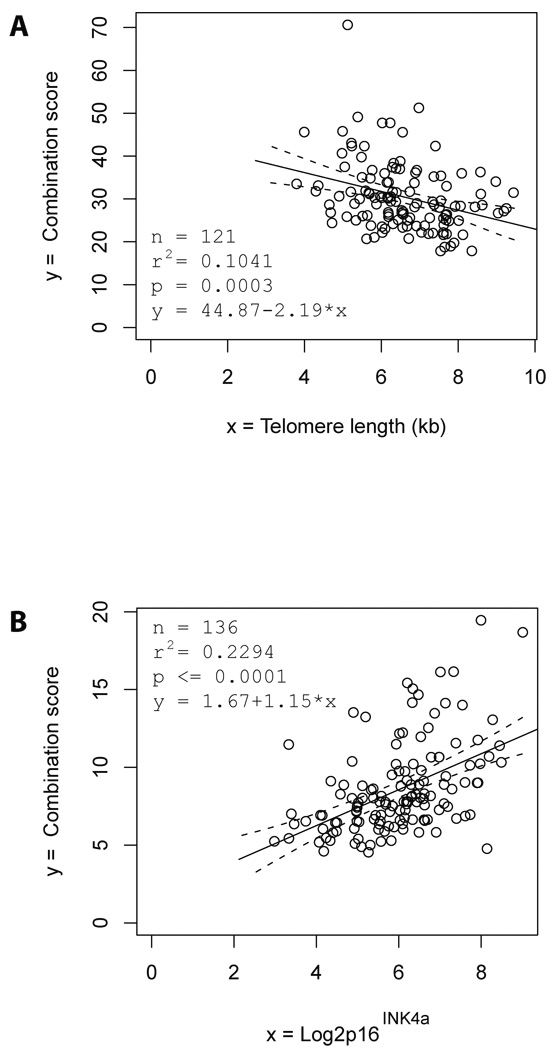
Individual serum markers of telomere dysfunction and DNA damage (except CRAMP) showed a significant association with the mRNA level of p16INK4a as well as to the telomere length in PBTLs (data not shown). The best biomarker combination showed a significant, inverse association with the telomere length (Fig. 5A and supplementary material) and a direct association with the mRNA expression level of p16INK4a (Fig. 5B, and supplementary material) of PBTLs.
Figure 5. The combination of serum markers of telomere dysfunction and DNA damage is directly associated with expression of P16 INK4a and inversely associated with telomere length.
Regression analysis showed that the combination of serum markers of telomere dysfunction and DNA damage is correlated with expression of P16 INK4a and telomere length. (A) the best combination score of serum markers of telomere dysfunction and DNA damage correlating to telomere length (0.1090 × Stathmin + 0.0948 × n-acetyl-glucosaminidase + 1.6531 × chitotriosidase), (B) the best combination score of serum markers of telomere dysfunction and DNA damage correlating to of P16 INK4a mRNA expression in blood lymphocytes (which equals to 1.7874 × chitibiosidase + 5.1279 × EF-1α). The black lines show the regression line and the dashed lines the 95% confidence bands for the regression line.
Discussion
The current study indicates that life style factors have an age-independent influence on the accumulation of biomarkers of DNA damage and telomere dysfunction in human blood serum. Previous studies have suggested that life style factors can influence on the accumulation of oxidative DNA damage, DNA breaks and DNA adduct formation (Niess et al. 1998; Tamae et al. 2009). Moreover, there are emerging reports that lifestyle interventions can reduce levels of DNA damage and telomere shortening in vivo (Allgayer et al. 2008; Hofer et al. 2008; O'Callaghan et al. 2009). There is growing evidence that the accumulation of DNA damage and telomere dysfunction can contribute to the impairments in organ maintenance and function during human aging and the progression of chronic diseases (for review see Nalapareddy et al. 2008). However, the development of biomarkers that can easily be measured is currently one of the biggest hurdles for a systematic evaluation of the prospective value of levels of DNA damage and telomere dysfunction in predicting the evolution of pathophysiological conditions and disease stages during human aging and chronic diseases. In this regard, the development of blood biomarkers of telomere dysfunction and DNA damage could provide a useful tool. The predictive and diagnostic value of the blood biomarkers needs to be evaluated in clinical trials and lifestyle intervention studies.
Our previous work has shown that CRAMP, EF-1α, Stathmin, and chitinase enzyme activity are induced in response to telomere dysfunction and DNA damage and these markers show an age dependent increase in human blood (Jiang et al. 2008). The current study shows that smoking and elevated body mass correlate with increased expression of the blood biomarkers of DNA damage, whereas exercise is correlated with reduced levels of expression. However, in most of the tested variables only a subset of the serum biomarkers was induced. A possible explanation indicates that different types of DNA lesion may be induced by different lifestyle factors and may thus lead to a differential induction of different subtypes of markers (Niess et al. 1998, Ramsey et al. 1995; Toyooka & Ibuki 2009). It is also possible that the blood biomarkers that were used in this study are induced by other cellular stresses that are not directly linked to DNA damage or telomere dysfunction, e.g. protein oxidation or mitochondria damage. However, the current study shows that the expression of serum markers of DNA damage correlated with two prominent markers of cellular senescence in PBTLs (telomere shortening and up-regulation of p16INK4a mRNA expression). These data support the concept that the blood markers represent biomarkers for the determination of telomere dysfunction and DNA damage – two well known inductors of cellular senescence.
To our knowledge, the here described biomarkers represent the first protein markers of DNA damage and telomere dysfunction that can easily be detected in serum and show a strong correlation with markers of cellular senescence. The technical difficulties in the in vivo determination of senescence, telomere dysfunction and DNA damage have hampered its use in clinical trials and lifestyle intervention studies. The current study indicates that such studies could be conducted by the use of novel serum markers of telomere dysfunction and DNA damage. Such studies could boost the performance of clinical and lifestyle intervention trials aiming to reduce the accumulation of telomere dysfunction and DNA damage in order to prevent age related declines in cellular and organismal functions.
Experimental procedures
Study subjects
Blood serum was obtained from healthy volunteers at a central site on the campus of the University of North Carolina Hospitals in Chapel Hill, NC. In addition, each volunteer completed a questionnaire about their health, health behaviors such as smoking and exercise, and demographics. Expression of p16INK4a was determined as previously described (Liu et al. 2009). Genomic DNA was prepared from buffy coats using Puregene Blood Core Kit (Qiagen) according to manufacture’s instructions. De-identified serum and DNA samples were sent to Ulm for analysis by investigators blinded to sample identification. All studies on human samples were carried out in accordance to the declaration of Helsinki and approved by the UNC Institutional review board. Extraction of RNA was described previously (Liu et al. 2009). Briefly, RNA was prepared from PBTLs using RNeasy mini kit (Qiagen) according to manufacture's directions. One µg of purified RNA was used for reverse transcription with ImProm-II™ RT system (Promega) according to manufacture's instructions. Expression of p16INK4a mRNA was determined as previously described (Liu et al. 2009). Physical activity was determined by 3 questions: 1. How often do you exercise per week? 2. How long do you exercise each time? 3. How many hours do you exercise per month?
Enzyme-linked Immunosorbent Assay
The assay was previously described (Jiang et al. 2008). Stathmin and EF-1α serum levels were determined by ELISA. Briefly, microwell plates (NUNC) were coated with 100 µl serum (4 times dilution) and standard protein at 4°C over night. For Stathmin, a commercially available standard protein was used (EBP07115, Everest biotech). For EF-1α, serum from young healthy controls was used as a standard. After incubation, the coated plates were washed 3 times with PBS-Tween, and then incubated with the first antibodies for 2h at room temperature anti-Stathmin (Everest Biotech, EB07115, 1:1000) and anti-EF1-α (Millipore, 05–235, 1:1000). After washing (4x with PBS-Tween) the plates were coated with secondary antibodies (for EF-1α: anti-mouse HRP; for Stathmin: anti-goat HRP) and incubated at room temperature for 1h. After washing the plates 4 times with PBS-Tween, 100 µL of TMB peroxide-based substrate solution (R&D systems, DY999) was added to each well, 30 minutes later 50 µL stop solution (R&D systems, DY994) was added to stop the reaction. The absorbencies were read in the microplate reader in dual-wavelength mode (450–540 nm). Human CRAMP levels were determined with the ELISA kit from Cell Sciences (Cat. #HK321) according to manufacture’s instructions.
Chitinase activity
Chitinase activity was measured with the Chitinase assay kit (Sigma, CS1030) using 2µl of serum per reaction. The chitinase assay kit was used to detect N-acetyl-glucosaminidase, chitobiosidase and chitotriosidase activity. The assay was performed according to manufacture’s instructions.
Quantitative realtime-PCR for telomere length
DNA was prepared from peripheral blood T-lymphocytes (PBTLs) using Puregene Blood Core Kit (Qiagen) according to the manufactures’ instructions. Quantitative RT-PCR was performed according to previously published method (Cawthon 2002). The relative telomere length was calculated with slight modifications according to the previously published method (Cawthon 2002). Briefly, 9 human cell lines were used as reference DNA samples. The mean telomere length of these cell lines was determined by Southern Blot (see supplementary information, page 5). The same reference DNA was analyzed by quantitative PCR to detect telomeric sequences (T) and a single copy gene (S), which was β globin gene (HBG). The correlation of the mean telomere length determined by Souther blotting with the T/S ratio was determined. These results were used as y standard for telomere length measurement of the test samples (see supplementary data page 5). Quantitative RT-PCR assay was performed on 96-well PCR plates., in a total volume of 25µl. Genomic DNA (20ng) was mixed with iTaq SYBR Green supermix (Bio-Rad) and 100nM forward and 900nM reverse primers for telomeres and, in a separate well. For HBG as single copy gene, the reaction final concentration was 300nM for forward primer and 700nM for reverse primer. The reaction was run for 40 cycles at 60°C in an RT-PCR cycler system (7300 Real Time PCR System, Applied Biosystems). Primer sequences were for telomeric DNA: fw 5’CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3’, rv 5’-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3’; for HBG: fw 5’-GCTTCTGACACAACTGTGTTCACTAGC-3’, rv 5’-CACCAACTTCATCCA CGTTCACC-3’.
Statistical methods
Statistical analyses were performed with Statistica (v. 7) and R (version 2.10, www.r-project.org). Simple and multiple linear regression analyses were performed using the standard least squares estimator. Best combination scores were calculated from the set of serum markers: n-acetyl-glucosaminide, chitobiosidase, chitotriosidase, Stathmin, CRAMP, and EF-1α. A population-based stochastic search method (particle swarm optimization) algorithm was used to maximise the rank correlation of a linear combination of all marker subsets under the non-negative weight constraint. See supplementary material for details (page 1–3).
Supplementary Material
Acknowledgment
The authors would like to thank Norman E. Sharpless for providing samples and expertise. This work was supported from the European Community's 7th Framework Programme (FP7/2007–2013) under grant agreement n° 202230, acronym “GENINCA”, the EU-TELOMARKER consortium, and the Deutsche Forschungsgemeinschaft (DFG) (RU745-13-1, RU745-10-1). Y.L, C.T, and P.D were funded from NIA grant AG024379. HK was funded by is supported by the German Science Foundation (SFB 518, Project C5), the Stifterverband für die Deutsche Wissenschaft (HAK), the German Federal Ministry of Education and Research (HAK) and the Graduate School of Mathematical Analysis of Evolution, Information and Complexity (HAK). This project was partly funded by the federal ministry of education and research (BMBF) within the framework of the program of medical genome research (PaCa-Net; project ID PKB-01GS08). The responsibility for the content lies exclusively with the authors.
References
- Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgayer H, Owen RW, Nair J, Spiegelhalder B, Streit J, Reichel C, Bartsch H. Short-term moderate exercise programs reduce oxidative DNA damage as determined by high-performance liquid chromatography-electrospray ionization-mass spectrometry in patients with colorectal carcinoma following primary treatment. Scand J Gastroenterol. 2008;43:971–978. doi: 10.1080/00365520701766111. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Chicca MC, Nesti C, Muzzoli M, Pasetti P, Pinamonti S. Correlation between age and DNA damage detected by FADU in human peripheral blood lymphocytes. Mutation research. 1996;316:201–208. doi: 10.1016/s0921-8734(96)90004-1. [DOI] [PubMed] [Google Scholar]
- Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang C, Buer J, Lee HW, von Zglinicki T, Ganser A, Schirmacher P, Nakauchi H, Rudolph KL. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nature genetics. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- Epel E, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34–53. doi: 10.1111/j.1749-6632.2009.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Dolbier C, Mendes WB, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008;28:1379–1384. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Bessler M, Mason PJ. Dyskerin, telomerase and the DNA damage response. Cell Cycle. 2009;8:6–10. doi: 10.4161/cc.8.1.7265. [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol Cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- Hofer T, Fontana L, Anton SD, Weiss EP, Villareal D, Malayappan B, Leeuwenburgh C. Long-term effects of caloric restriction or exercise on DNA and RNA oxidation levels in white blood cells and urine in humans. Rejuvenation Res. 2008;11:793–799. doi: 10.1089/rej.2008.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer T, Karlsson HL, Moller L. DNA oxidative damage and strand breaks in young healthy individuals: a gender difference and the role of life style factors. Free Radic Res. 2006;40:707–714. doi: 10.1080/10715760500525807. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ju Z, Rudolph KL. Telomere shortening and ageing. Z Gerontol Geriatr. 2007;40:314–324. doi: 10.1007/s00391-007-0480-0. [DOI] [PubMed] [Google Scholar]
- Jiang H, Schiffer E, Song Z, Wang J, Zurbig P, Thedieck K, Moes S, Bantel H, Saal N, Jantos J, Brecht M, Jeno P, Hall MN, Hager K, Manns MP, Hecker H, Ganser A, Dohner K, Bartke A, Meissner C, Mischak H, Ju Z, Rudolph KL. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc Natl Acad Sci U S A. 2008;105:11299–11304. doi: 10.1073/pnas.0801457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- Lieber MR, Karanjawala ZE. Ageing, repetitive genomes and DNA damage. Nat Rev Mol Cell Biol. 2004;5:69–75. doi: 10.1038/nrm1281. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, Thomas NE, Sharpless NE. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–448. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabello L, Huang WY, Wong JY, Chatterjee N, Reding D, Crawford ED, De Vivo I, Hayes RB, Savage SA. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8:405–413. doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoue T, Tokunaga S, Kasai H, Kawai K, Sato M, Kubo T. Body mass index and oxidative DNA damage: a longitudinal study. Cancer Sci. 2007;98:1254–1258. doi: 10.1111/j.1349-7006.2007.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalapareddy K, Jiang H, Guachalla Gutierrez LM, Rudolph KL. Determining the influence of telomere dysfunction and DNA damage on stem and progenitor cell aging: what markers can we use? Experimental gerontology. 2008;43:998–1004. doi: 10.1016/j.exger.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Niess AM, Baumann M, Roecker K, Horstmann T, Mayer F, Dickhuth HH. Effects of intensive endurance exercise on DNA damage in leucocytes. J Sports Med Phys Fitness. 1998;38:111–115. [PubMed] [Google Scholar]
- O'Callaghan NJ, Clifton PM, Noakes M, Fenech M. Weight loss in obese men is associated with increased telomere length and decreased abasic sites in rectal mucosa. Rejuvenation Res. 2009;12:169–176. doi: 10.1089/rej.2008.0819. [DOI] [PubMed] [Google Scholar]
- Ramsey MJ, Moore DH, 2nd, Briner JF, Lee DA, Olsen L, Senft JR, Tucker JD. The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutation research. 1995;338:95–106. doi: 10.1016/0921-8734(95)00015-x. [DOI] [PubMed] [Google Scholar]
- Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Dürr P, Wlaschek M. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5:379–389. doi: 10.1111/j.1474-9726.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Rundle A, Madsen A, Orjuela M, Mooney L, Tang D, Kim M, Perera F. The association between benzo[a]pyrene-DNA adducts and body mass index, calorie intake and physical activity. Biomarkers. 2007;12:123–132. doi: 10.1080/13547500601010418. [DOI] [PubMed] [Google Scholar]
- Schaetzlein S, Kodandaramireddy NR, Ju Z, Lechel A, Stepczynska A, Lilli DR, Clark AB, Rudolph C, Kuhnel F, Wei K, Schlegelberger B, Schirmacher P, Kunkel TA, Greenberg RA, Edelmann W, Rudolph KL. Exonuclease-1 deletion impairs DNA damage signaling and prolongs lifespan of telomere-dysfunctional mice. Cell. 2007;130:863–877. doi: 10.1016/j.cell.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid TE, Eskenazi B, Baumgartner A, Marchetti F, Young S, Weldon R, Anderson D, Wyrobek AJ. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod. 2007;22:180–187. doi: 10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Redon C, Nakamura A, Zimonjic DB, Popescu NC, Bonner WM. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell. 2008;7:89–100. doi: 10.1111/j.1474-9726.2007.00354.x. [DOI] [PubMed] [Google Scholar]
- Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80:1420–1430. doi: 10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Stein GH, Drullinger LF, Soulard A, Dulić V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- Tamae K, Kawai K, Yamasaki S, Kawanami K, Ikeda M, Takahashi K, Miyamoto T, Kato N, Kasai H. Effect of age, smoking and other lifestyle factors on urinary 7-methylguanine and 8-hydroxydeoxyguanosine. Cancer Sci. 2009;100:715–721. doi: 10.1111/j.1349-7006.2009.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CA, Rock CL, Giuliano AR, Newton TR, Cui H, Reid PM, Green TL, Alberts DS. Longitudinal changes in body weight and body composition among women previously treated for breast cancer consuming a high-vegetable, fruit and fiber, low-fat diet. Eur J Nutr. 2005;44:18–25. doi: 10.1007/s00394-004-0487-x. [DOI] [PubMed] [Google Scholar]
- Toyooka T, Ibuki Y. Cigarette sidestream smoke induces phosphorylated histone H2AX. Mutation research. 2009;676:34–40. doi: 10.1016/j.mrgentox.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- Walne AJ, Dokal I. Advances in the understanding of dyskeratosis congenita. Br J Haematol. 2009;145:164–172. doi: 10.1111/j.1365-2141.2009.07598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Huang X, Jiang H, Zhang Y, Liu H, Qin C, Eisner GM, Jose PA, Rudolph L, Ju Z. Short telomeres and prognosis of hypertension in a chinese population. Hypertension. 2009;53:639–645. doi: 10.1161/HYPERTENSIONAHA.108.123752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn RK, Reinmuller J, Beyer R, Pondeljak V. Age-correlated DNA damage in human muscle tissue. Mech Ageing Dev. 1987;41:73–114. doi: 10.1016/0047-6374(87)90055-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.