Abstract
Transient Receptor Potential channels are polymodal cellular sensors involved in a wide variety of cellular processes, mainly by increasing cellular Ca2+. In this review we focus on the roles of these channels in: i) cell death ii) proliferation and differentiation and iii) synaptic vesicle release.
Cell death
Ca2+ influx participates in apoptotic and necrotic cell death. The Ca2+ permeability and high sensitivity of part of these channels to oxidative/metabolic stress make them important participants in cell death. Several examples are given. Transient Receptor Potential Melastatin 2 is activated by H2O2, inducing cell death through an increase in cellular Ca2+ and activation of Poly ADP-Ribose Polymerase. Exposure of cultured cortical neurons to oxygen-glucose deprivation, in vitro, causes cell death via cation influx, mediated by Transient Receptor Potential Melastatin 7. Metabolic stress constitutively activates the Ca2+ permeable Transient Receptor Potential channels of Drosophila photoreceptor in the dark, potentially leading to retinal degeneration. Similar sensitivity to metabolic stress characterizes several mammalian Transient Receptor Potential Canonical channels.
Proliferation and differentiation
The rise in cytosolic Ca2+ induces cell growth, differentiation and proliferation via activation of several transcription factors. Activation a variety of store operated and Transient Receptor Potential channels cause a rise in cytosolic Ca2+, making these channels components involved in proliferation and differentiation.
Synaptic vesicle release
Transient Receptor Potential Melastatin 7 channels reside in synaptic vesicles and regulate neurotransmitter release by a mechanism that is not entirely clear. All the above features of Transient Receptor Potential channels make them crucial components in important, sometimes conflicting, cellular processes that still need to be explored.
Keywords: TRP channels, cell death, proliferation and differentiation, synaptic vesicle release
1. Introduction
The TRP channel superfamily is a large and diverse group of cation channels. The founding member of the family was discovered due to a Drosophila mutant, which has a defect in its response to light (Cosens and Manning, 1969). This spontaneously formed mutant fly has a transient, rather than a sustained response to prolonged illumination, hence designated “TRP” – Transient Receptor Potential (Minke et al., 1975). After the cloning of the trp gene (Montell and Rubin, 1989), the missing protein in the trp mutant was found to be the main route of Ca2+ entry (Hardie and Minke, 1992; Peretz et al., 1994) and the channel which produces the voltage light response (Hardie & Minke, 1992). A second member of the family was discovered and designated “TRP-like” (TRPL (Phillips et al., 1992)), and these channels were established as a new type of cation channel (Hardie and Minke, 1993; Phillips et al., 1992). TRP and TRPL channels are expressed in the Drosophila photoreceptor cells and both constitute the light activated channels (Niemeyer et al., 1996).
Other channels, both in mammals and in invertebrates, were found to be part of the TRP superfamily on the basis of similarity to the TRP amino acid sequence, mainly in the transmembrane domain regions. The channels are classified into 7 subfamilies: TRPC, TRPM, TRPV, TRPA, TRPN, TRPML and TRPP (see reviews (Clapham, 2003; Montell, 2001; Montell, 2005; Nilius and Voets, 2005; Pedersen et al., 2005)). The TRPP and TRPML subfamilies form a distinct group in the TRP superfamily since their amino acid sequence have low similarity to the other TRP channels (Montell, 2005). Therefore, we will not discuss these subfamilies in this review. There is a significant variability in the primary amino acid sequence among the different subfamilies. Three features, typically characterizing several types of voltage gated and CNG channels, are present in all TRPs: the permeability to cations, the basic architecture of six transmembrane segments, S1–S6, with a pore region between S5 and S6 and subunit organization as tetramers. The majority of the TRP channels function both as homotetramers and heterotetramers. Heterotetrameres are mostly found within the same subfamily, although there are a few exceptions (Bai et al., 2008; Kottgen et al., 2008; Ma et al., 2010). Other structural characteristics of the TRP channels are: ankyrine repeats, TRP box (WKFQR motif), proline rich region, calmodulin binding site, PDZ-binding motif (amino acid VTTRL), coiled coil domain and partial pleckstrin homology domain (reviewed by (Ramsey et al., 2006)). These features are present in many but not all TRP channels.
Most of the knowledge on the characteristics of TRP channels was obtained by studying heterologously-expressed channels. Therefore, relatively little is known about the physiological role of TRP channels in the native cells. However, additional studies have been recently performed in vivo or in primary cell cultures, and therefore the knowledge and understanding of TRP channels in the physiological relevant context is expanding.
A major difficulty in the study of TRP channels is that most channels do not have specific pharmacological agents which can activate or inhibit the channels. Since most cells express more than one member of the TRP family, the distinction between the characteristics and function of the individual channels is not clear. Thus, there is a need to use a genetic approach rather than a pharmacological one. However, there are several drawbacks to the genetic approach as well. It seems that there are interactions between the regulatory mechanisms underlying the expression of different channels within the same TRP subfamily. In some cases, down-regulation of one TRP family member can result in down or up-regulation of another TRP member, thus making it difficult to determine which channel is responsible for the specific effect. In addition, compensation by another TRP channel can mask the deficiency in the examined channel. This is illustrated for the TRPC6 knockout mice (TRPC6−/−), in which it was suggested that for some functional processes, TRPC3 up-regulation compensates for TRPC6 loss (Dietrich et al., 2005). Additionally, knocking down one TRP family member may affect the physiological balance between the different TRP channels within a cell, which might result in formation of heteromultimers that do not usually exist, thus masking the isolated effect of one channel. These drawbacks mainly characterize mammalian TRP channels because many mammalian cells express several TRPs of the same subfamily in single cells as exemplified above. This complication is largely reduced in invertebrate species used for genetic dissection of various functions such as C. elegans and Drosophila melanogaster. In these invertebrates species, the redundancy of TRP channel activity is largely avoided due to the expression of only few members of a specific subfamily in single cells and because of their molecular-genetic power that allows easy separation between the function of different channels.
Like the founding member of the TRP channels, which mediates phototransduction, many TRPs have also been found to participate in sensory transduction pathways, including thermosensation, mechanosensation, taste perception, perception of pungent compounds, pheromone sensing, osmolarity and pain sensation (for reviews see (Clapham, 2003; Minke and Cook, 2002; Montell, 2001; Nilius & Voets, 2005)). Apart from sensory perception, the involvement of TRP channels was demonstrated in many other processes, including salivary fluid secretion, inflammation, cardiovascular regulation, smooth muscle tone, pressure regulation, Ca2+ and Mg2+ homeostasis and lysosomal function. In addition, TRP channels were shown to be involved in many cellular functions, including cell adhesion, control of growth and differentiation, proliferation, cell death and cell polarity (Abramowitz and Birnbaumer, 2009; Miller, 2006; Nishida et al., 2006).
Most of the reviews on TRP channels have been focused on their sensory functions and ion transport. In the present review we focus on several cellular functions involving TRP channels, including cell death, proliferation, differentiation and neurotransmitter release. A question arises regarding the specific features of TRP channels, which allow them play important roles in the above cellular functions. Some of these TRP specific properties are described below.
One of the most important properties of TRP channels is their permeability to Ca2+. Ca2+ is a ubiquitous intracellular signaling ion, involved in the regulation of many distinct cellular processes. Elevation in intracellular Ca2+ concentration is mediated by two main pathways: Ca2+ influx from the extracellular medium into the cell and Ca2+ release from intracellular stores. Most TRP channels are localized and function at the plasma membrane. Except for the mammalian TRPM4 and TRPM5, almost all TRP channels are Ca2+ permeable, although the Ca2+ permeability differs from one channel to another.
In addition to their permeability to Ca2+, the Drosophila TRP channels have additional important properties, which also characterize several mammalian TRP channels. These properties include organization in multimolecular signaling complexes, protein-protein interactions and signal induced translocation between the surface plasma membrane and cytoplasmatic vesicles (for a review see (Minke and Parnas, 2006)). The organization of the Drosophila photoreceptor’s signaling proteins in a specific cellular compartment in the form of a multimolecular signaling complex seems to be a general mechanism (Minke & Parnas, 2006). Studies in mammalian cells showed that the adaptor protein termed, Homer, facilitates a physical association between TRPC1 and the InsP3 receptor that is required for the TRPC1 channel to respond to signals. The TRPC1-Homer-InsP3R complex is dynamic and its disassembly parallels TRPC1 activation (Yuan et al., 2003). In other studies, the mammalian TRPC3 has been found in a specific microdomain called caveoli. TRPC3 constitutes a part of a multimolecular signaling complex containing Ezrin and key Ca2+ signaling proteins including PLCβ1 and Gαq/11, which are involved in Ca2+-mediated regulation of TRPC3 channel activity and cytoskeletal reorganization (Lockwich et al., 2001). Another study reveals that PLC-γ 1 binds to and regulates TRPC3 channels while this interaction requires a partial pleckstrin homology (PH) domain of PLC-γ 1 and TRPC3 (van Rossum et al., 2005). Another study has shown that the first PDZ domain of the Ezrin Radixin Moesin (ERM) adaptor and the Na+/H+ exchanger regulatory factor (NHERF, or EBP50) associate with PLCβ, TRPC4 and TRPC5, and regulate channel activity and sub-cellular localization (Mery et al., 2002; Obukhov and Nowycky, 2004). These data strongly suggest that multimolecular interactions found in Drosophila TRP are evolutionarily conserved mechanisms, with important functional roles.
An additional striking property of the Drosophila TRPL channel is its reversible light induced translocation from the signaling surface membrane to intracellular compartments. This property is specific to TRPL and was not observed for TRP. The signal induced modulation of Ca2+ permeable channel level at the surface membrane modulates Ca2+ influx and has functional consequences (Bahner et al., 2002). Signal induced translocation between the plasma membrane and intracellular vesicles characterize many mammalian TRP channels. Growth factor stimulation initiated rapid (~2min) incorporation into the plasma membrane in HEK-293 cells, of expressed TRPC5 from vesicles held in reserve, just under the plasma membrane. This incorporation was specific to TRPC5 and was not observed for TRPC1 (Bezzerides et al., 2004). Strikingly, TRPC5 incorporation into the plasma membrane resulted in a dramatic increase in the typical current produced by these channels (Bezzerides et al., 2004), indicating insertion of functional channels in similar manner to the Drosophila TRPL (Bahner et al., 2002).
Internalization of TRPC1, together with its signaling complex Gq/11, PLCβ1 and caveolin-1, was reported to occur following treatment with the protein phosphatase inhibitor caliculin A, which also induced reorganization of the actin cytoskeleton (Lockwich et al., 2001). This channel internalization led to a marked attenuation in divalent cation (Sr2+) entry induced by application of OAG, reminiscent of the reduction of TRPL dependent currents in Drosophila upon translocation of TRPL. Stimulation of the M3 muscarinic receptor resulted in translocation of TRPC6 to the plasma membrane in a timescale that coincided with activation by Ca2+ influx (Cayouette et al., 2004).
Translocation of another mammalian TRP channel (TRPV2) was reported for Balb/c 3T3 cells. These cells express the TRPV2 channel which is regulated by the insulin-like growth factor (IGF-1). Interestingly, stimulation of the cells by IGF-1 induced translocation of the channels from internal vesicles to the plasma membrane (Kanzaki et al., 1999). Similar experiments with heterologously expressed TRPV1 did not reveal any translocation (Bezzerides et al., 2004). Interestingly, TRPV2 as well as IGF-1 and its receptor are over-expressed in bladder cancer. Increased proliferation and survival of human bladder smooth muscle cells induced by mechanical stress is associated with increased IGF-1 levels (Zhao et al., 2003). These studies suggest a relationship between TRPV2 translocation and cellular proliferation.
The following properties which include: permeability to Ca2+, protein-protein interactions, organization in multimolecular signaling complexes and signal induced translocation characterize TRP channels of both Drosophila and mammalian cells. These properties of TRP channels emerge as important regulatory mechanisms with wide implications for a variety of cellular functions. Below we review cellular functions of TRP channels in light of these properties by highlighting several examples from the various TRP channels. The involvement in cellular functions of the various TRP channels outlined in the review is summarized in Table 1.
Table 1.
The involvement in cellular functions of the various TRP channels outlined in the review
| Function | Channel | Cellular action | Tissue | Reference |
|---|---|---|---|---|
| Cell death | TRPM2 | TRPM2-antisense oligonucleotide suppressed the H2O2/TNF-α-induced Ca2+ elevation and cell death. BAPTA prevented cell death induced by H2O2. |
Rat insulinoma β-cell line RIN-5F Monocyte cell line U937 |
(Hara et al., 2002) |
| TRPM2-siRNA suppressed the H2O2 -induces Ca2+ elevation and cell death. | Rat cultured cortical neurons | (Kaneko et al., 2006) | ||
| TRPM2-S, a dominant negative isoforms of TRPM2, inhibited the H2O2 and Amyloid β-peptide-induces Ca2+ elevation and cell death. PARP inhibitors attenuated H2O2 induced cell death. |
Rat cultured striatal cells | (Fonfria et al., 2005) | ||
| Cell death induced by H2O2 was shown to be mediated both by plasma membrane localized TRPM2 and lysosomal localized TRPM2 channels. | Rat insulinoma β-cell line INS-1 | (Lange et al., 2009) | ||
| H2O2 Increased PARP/procaspases -8, -9, -7, and -3 cleavage. BAPTA blocked cell death induced by TRPM2 activation. |
Human monocytic U937-ecoR cells | (Zhang et al., 2006) | ||
| TRPM7 | Prolonged OGD elicited ROS production which induced TRPM7 activation and cell death. Suppressing TRPM7 expression with siRNA prevented Ca2+ entry, ROS production and cell death. |
Cultured cortical neurons | (Aarts et al., 2003) | |
| Suppression of TRPM7 expression decreased delayed neuronal death following transient global cereberal ischemia. | Rat hippocampal neurons (in vivo) | (Sun et al., 2009) | ||
| Drosophila TRP | Cells expressing constitutively active mutant channel (trpP365) display severe degeneration. | Drosophila photoreceptor cells (in vivo) | (Yoon et al., 2000) | |
| TRPC1 | Overexpression of TRPC1 inhibited NFκB activity and increased susceptibility to apoptosis, induced by TNF-α together with cyclohexamide or staurosporine. TRPC1-siRNA treatment prevented the increased in susceptibility to apoptosis. | IEC-6 intestinal epithelial cells | (Marasa et al., 2006) | |
| TRPC3 | High level of apoptosis was observed in response to ischemia- reperfusion treatment in cells overexpressing TRPC3. | Cultured mice cardiomyocytes | (Shan et al., 2008) | |
| TRPC7 | Increased intracellular Ca2+ and basal & angiotensin II-induced- apoptosis was observed in cells overexpressing TRPC7. | Cultured neonatal rat cardiomyocytes | (Satoh et al., 2007) | |
| Differentiation | TRPC1 | TRPC1 expression was significantly upregulated during myogenesis. | Murine C2C12 skeletal myoblasts | (Formigli et al., 2009) |
| TRPC1-siRNA treatment decreased calcium induced differentiation. | Human gingival keratinocytes | (Cai et al., 2006) | ||
| TRPC1 & TRPC3 | Increased TRPC1 and TRPC3 expression was observed under differentiating conditions. TRPC1 or TRPC3-siRNA treatment inhibited SOCE and differentiation. | Hippocampal cells H19-7 | (Wu et al., 2004) | |
| TRPC1 & TRPC4 | TRPC1/TRPC4-siRNA treatment prevented calcium induced differentiation. | Human keratinocyte cell line HaCaT | (Beck et al., 2008) | |
| TRPV4 | mRNA levels of TRPV4 was increased in correlation with osteoclast maturation. TRPV4 was found to be responsible for the sustained Ca2+ influx, which is necessary for terminal differentiation of osteoclast. | Cultured osteoclasts isolated from TRPV4−/− or control mice | (Masuyama et al., 2008) | |
| TRPV4 induced increased activation of the SOX9 (essential transcription factor for chondrocytes differentiation) promoter. | Murine chondrogenic cell line, ATDC5 | (Muramatsu et al., 2007). | ||
| TRPV6 | Increased TRPV6 expression was observed upon induction of differentiation. TRPV6-siRNA treated cells showed decreased expression of specific-differentiation markers. | Human primary keratinocytes | (Lehen’kyi et al., 2007a) | |
| Proliferation | TRPC1 | These cells showed increased TRPC1 expression and enhanced proliferation. | Keratinocyte from Darier’s disease patients | (Pani et al., 2006) |
| Antisense knock-down of TRPC1 protein led to decreased bFGF- mediated Ca2+ influx and proliferation. | Embryonic rat neural stem cells | (Fiorio et al., 2005) | ||
| TRPC1-siRNA treatment inhibited platelet-derived growth factor- induced proliferation. | Human osteoblastic cells MG-63 Murine osteoblast cell line MC3T3 |
(Abed et al., 2009) | ||
| Proliferative cells showed upregulation in TRPC1 expression. TRPC1- antisense oligonucleotide suppressed the SOC current and decreased cell growth rate. | Primary cultured PASMC | (Golovina et al., 2001; Sweeney et al., 2002) | ||
| Overexpression of caveolin-1 prevented TRPC1-induced proliferation and overexpression of STIM1 enhanced the proliferation. | Human submandibular gland cells | (Pani et al., 2009) | ||
| TRPC1 & TRPC4 | TRPC1 and TRPC4 shRNA inhibited neurite extention. | Human embryonic stem cells | (Weick et al., 2009) | |
| TRPC4 | TRPC4-siRNA attenuated ATP-induced proliferation. Nonphosphorylatable CREB mutant prevented both the increased TRPC4 expression and proliferation. | Primary cultured PASMC | (Zhang et al., 2004) | |
| TRPC6 | PDGF induced an increase in TRPC6 expression, SOC current and proliferation. c-jun downregulation attenuated the increased TRPC6 expression. TRPC6 - antisense oligonucleotides reduced SOC current and PDGF-mediated proliferation. | Primary cultured PASMC | (Yu et al., 2003) | |
| TRPC6-siRNA attenuated proliferation. | PASMCs from patients with idiopathic pulmonary arterial hypertension | (Yu et al., 2004). | ||
| Overexpression of TRPC6 resulted in an increase of cell proliferation rate while knockdown of TRPC6 expression had the opposite effect. | Human hepatoma cell lines Huh- 7 and HepG2 | (El et al., 2008) | ||
| Stimulation of the α1-AR resulted in increased proliferation together with increased TRPC6 and CDK4 expression, NFAT activation and decreased p27 expression. TRPC6 antisense nucleotide inhibited proliferation. | Primary human prostate cancer epithelial cells | (Thebault et al., 2006) | ||
| Hypoxia treatment induced aggressive growth, activation of the Notch signaling, increased TRPC6 expression, intracellular Ca2+ and NFAT activation. TRPC6-siRNA prevented the aggressive glioma growth and invasion, Ca2+ elevation and NFAT activation. | Primary cells and cell lines derived from glioblastoma multiforme | (Chigurupati et al., 2010) | ||
| Mice expressing activated calcineurin displayed heart hypertrophy and elevated TRPC6 expression. Overexpression of TRPC6 in transgenic mice induced NFAT activation. | Mice cardiomyocytes (in vivo) | (Kuwahara et al., 2006) | ||
| Inhibition of TRPC6 activity by the dominant negative isoform, DNC6, inhibited cell proliferation. TRPC6 was found to be required for tumor growth in vivo. | Gastric cancer cells AGS and MKN45 in vivo experiment in nude mice |
(Cai et al., 2009) | ||
| inhibition of TRPC6 activity by the dominant negative isoform, DNC6, inhibits VEGF induced proliferation. | Human umbilical vein endothelial cells | (Ge et al., 2009) | ||
| TRPM7 | TRPM7-siRNA suppressed the angiotensin II-induced-elevation in intracellular Mg2+ concentration, and cell growth. | Vascular smooth muscle cells | (He et al., 2005) | |
| TRPM7-siRNA treatment inhibited both basal and platelet-derived growth factor-induced proliferation | Human osteoblastic cells MG-63 Murine osteoblast cell line, MC3T3 |
(Abed et al., 2009) | ||
| TRPM7-siRNA reduced cell proliferation via an inhibition of the G1 to S phase transition in the cell cycle. | Retinoblastoma cells | (Hanano et al., 2004) | ||
| TRPM8 | Higher transcript levels of TRPM8 was observed in malignant relative to non-malignant tissues, with correlation to tumor stages | Human primary prostate carcinoma | (Fuessel et al., 2003) | |
| TRPV6 | TRPV6 was up-regulated in prostate cancer, in correlation with the tumor grade and aggressiveness. | Human prostate tissue | (Fixemer et al., 2003; Wissenbach et al., 2001) | |
| TRPV6-siRNA inhibited cell proliferation rate. | Human prostate cancer cell line, LNCaP | (Lehen’kyi et al., 2007b) | ||
| Elevated TRPV6 level was observed in prostate, breast, thyroid, colon, and ovarian carcinomas, in comparison with normal tissues. | (Zhuang et al., 2002) | |||
| Transmitter release | TRPM7 | TRPM7 is localized to the synaptic vesicles and interacts with snapsin, synapsin I and synaptotagmin. The amplitudes, quantal sizes, and decay times of the EPSPs correlated with TRPM7 expression levels. Dominant-negative TRPM7 mutant suppressed the postsynaptic responses. TRPM7 specific siRNA inhibited vesicles fusion. |
Primary rat superior cervical ganglion neurons PC12 |
(Krapivinsky et al., 2006) (Brauchi et al., 2008) |
2. Cell death
Cation influx is required for several mechanisms of cell death. In particular, Ca2+ is an important factor in apoptotic and necrotic cell death (Orrenius et al., 2003). Several cation channels have been shown to participate in cell death, including the L-type voltage gated calcium channel and the NMDA channel. Several TRP channels are unique in the sense that they combine two important properties, which make cells expressing these channels vulnerable to death. These properties are: activation or regulation by oxidative or metabolic stress, combined with Ca2+ permeability. Oxidants, apart from activating several TRP channels, can reverse the activity of Na+/Ca2+ exchangers and repress the activity of pumps, thus enhancing the increase of intracellular Ca2+ (Ermak and Davies, 2002). In this section we review the involvement of specific TRP channels in cell death with emphasis on the properties of TRP channels described in the Introduction.
2.1 Transient Receptor Potential Melastatin 2 (TRPM2)
TRPM2 is a Ca2+ permeable non selective cation channel, with a linear current-voltage relationship. The channel is expressed in the brain, hematopoietic cells, the intestine and pancreatic β-cells (Harteneck, 2005). The trpm2 gene was cloned in 1998 (Nagamine et al., 1998) and initially termed trpc7. After the establishment of a unified nomenclature for the TRP channels, this channel was affiliated to the TRPM subfamily and was renamed as TRPM2 (Montell et al., 2002). In addition to the common structure of six transmembrane segments, the channel has a unique nudix domain at the C terminus. This domain is a consensus region for pyrophosphatases, although the channel has very low enzymatic activity in vitro (Perraud et al., 2001). Rather, this region constitutes the binding site of ADP-ribose (ADPR), which is the physiological endogenous activator of the channel (Kuhn and Luckhoff, 2004).
Apart from the physiological activator, ADPR, which activates TRPM2 when added to the intracellular solution, cADPR and NAD+ were also found to activate the channel intracellularly, although cADPR only facilitates activation by ADPR, and NAD+ might activate the channel indirectly, as a precursor of ADPR (Kolisek et al., 2005; Perraud et al., 2001; Sano et al., 2001). Tumor necrosis factor-α (TNFα) and low concentrations of H2O2 in the extracellular solution activate the channel (Hara et al., 2002; Wehage et al., 2002). Although one study concludes that H2O2 activation does not involve ADPR (Wehage et al., 2002), other studies support a model by which oxidative stress, induced by H2O2, leads to the production of ADPR in the mitochondria, which is then released to the cytosol and activates the channel (Perraud et al., 2005). Cytosolic Ca2+ was shown to be an essential positive modulator of TRPM2 (McHugh et al., 2003; Starkus et al., 2007), most likely via calmodulin (Starkus et al., 2007; Tong et al., 2006). A recent study reports that a high intracellular Ca2+ concentration is sufficient to activate the channel with no requirement for exogenous ADPR (Du et al., 2009). Furthermore, it was shown that a high intracellular Ca2+ concentration is capable of activating alternative spliced isoform of TRPM2, which is insensitive to ADPR. However, in resting cells, physiological intracellular Ca2+ concentrations cannot activate TRPM2, since activation requires high Ca2+ concentrations (in the μM range).
The first evidence for the involvement of TRPM2 in cell death was obtained in HEK293 cells, heterologously expressing the TRPM2 channel. Several groups showed that oxidative stress induced a rise in intracellular Ca2+ and raised the cells susceptibility to death. TRPM2-S, which is a native dominant negative isoform of TRPM2, inhibits both the rise in Ca2+ and the susceptibility to cell death (Zhang et al., 2003). Studies were also carried out on native cells, which express TRPM2 endogenously. Hara and colleagues used the rat insulinoma β-cell line, RIN-5F, and the monocyte cell line U937, and showed that H2O2 induces Ca2+ elevation and cell death. Both effects were suppressed when the cells were treated with antisense oligonucleotide, which reduced TRPM2 expression (Hara et al., 2002). These results were repeated in cultured cortical neurons and striatal cells, and the specific involvement of TRPM2 was shown using siRNA targeted to TRPM2, and the dominant negative isoform of TRPM2, S-TRPM2, respectively (Kaneko et al., 2006) (Fonfria et al., 2005). A new finding revealed localization of TRPM2 channels to lysosomal vesicles in the β-cell line INS-1 (Lange et al., 2009). Cell death induced by H2O2 was shown to be mediated both by plasma membrane localized TRPM2 and lysosomal localized TRPM2 channels. Since elimination of TRPM2 channels from only one compartment is not feasible, Ca2+ free extracellular solution was used to prevent TRPM2-Ca2+ influx through the plasma membrane. H2O2 induced cell death in Ca2+ free extracellular solution, indicating that cell death is mediated by lysosomal TRPM2 channels. Na+ Influx induced by oxidative stress has been shown to be involved in necrotic cell death (Carini et al., 1999). Thus, plasma membrane-TRPM2 channels might also be involved in TRPM2-induced cell death via Na+ influx.
The above experiments were carried out in a variety of tissues, demonstrating the wide spread and general involvement of TRPM2 in cell death. However, the mechanism by which TRPM2 activation by H2O2 leads to cell death is still not entirely clear. The different cell lines and techniques used by various groups led to different conclusion, suggesting that both apoptosis and necrosis are involved in cell death pathways. Nevertheless, one cannot exclude the possibility that both pathways may take place within the same cell line. Ca2+ is apparently a critical player in the TRPM2-induced cell death, since several groups reported an increase in intracellular Ca2+ concentrations following H2O2 application. In addition, preventing the increase in intracellular Ca2+ following H2O2 application with the Ca2+ chelator, BAPTA, inhibited cell death in TRPM2 expressing U937-ecoR cells (Zhang et al., 2006). Poly ADP-Ribose Polymerase (PARP) seems to participate in TRPM2-induced cell death as well. PARP inhibitors decreased both H2O2 induced elevation of intracellular Ca2+ and the loss in membrane integrity, in TRPM2 expressing HEK293 cells and in insulinoma CRI-G1 cells (Fonfria et al., 2004; Perraud et al., 2005). PARP inhibitors also attenuated H2O2 induced cell death in rat striatal cells (Fonfria et al., 2005). Conflicting results were provided by Zhang and colleagues, showing increased PARP cleavage, as well as increased cleavage of procaspases-8, -9, -7, and -3 in response to H2O2 application in the U937-ecoR cell line (Zhang et al., 2006). The authors explain this discrepancy by suggesting a feedback loop, in which TRPM2 is activated by PARP, but TRPM2 activation results in PARP cleavage and inactivation. PARP is activated in response to DNA damage that can be induced by oxidative stress and necrotic stimuli. It is well known that PARP cleavage and inactivation, as well as caspases activation are features of apoptosis (Pieper et al., 1999; Saraste and Pulkki, 2000).
In summary, TRPM2 is clearly involved in cell death and the still unclear underlying mechanism seems to involve PARP activation and mainly an increase in cellular Ca2+ due to opening of the Ca2+ permeable TRPM2 channels, both at the plasma membrane and in lysosomal membrane.
2.2 Transient Receptor Potential Melastatin 7 (TRPM7)
TRPM7 is a cation channel, which conducts both monovalent and divalent cations, but with a high selectivity to divalent cations. The channel is permeable not only to Ca2+ and Mg2+, but also to other divalent cations, such as Zn2+, Ni2+ and Ba2+ (Monteilh-Zoller et al., 2003). The channel displays an outwardly rectifying current-voltage relationship, in which the small inward current is carried exclusively by divalent cations. After the removal of divalent cations from the extracellular solution, the channel conducts monovalent cations and a linear current-voltage relationship of the channel is revealed (Nadler et al., 2001). Thus, the outwardly rectifying I-V curve is most likely due to divalent cation open channel block (Parnas et al., 2009).
The TRPM7 channel was discovered independently by two groups, using two different approaches. One group identified the channel in a yeast two hybrid screen using the C2 domain of phospholipase C (PLC) as bait, hence, designating the channel TRP phospholipase C interacting kinase, (TRP-PLIK (Runnels et al., 2001)). The other group identified the channel using a bioinformatics approach aimed at identifying novel ion channels expressed in haematopoietic cells (Nadler et al., 2001). The channel has an MHCK/EEF2-alpha kinase homology domain and therefore functions as ion channel and a protein kinase, a unique property for a channel protein that may account for its diverse, sometimes contradictory functions.
TRPM7 has little constitutive activity in both heterologus systems and native cells. High Mg2+ concentrations, either free or nucleotide-bound-Mg2+, inhibit the channel (Nadler et al., 2001). There is no agreement regarding the involvement of the kinase domain in channel regulation. It seems that the kinase domain does not have an essential role in TRPM7 activation but can modulate channel activity and may be required for its diverse functions ( see also review by (Penner and Fleig, 2007)). The channel seems to be regulated by phosphatidylinositol 4,5 bisphosphate (PIP2), although this issue is still controversial (reviewed by (Penner & Fleig, 2007)).
TRPM7 is ubiquitously expressed in almost every tissue and organ (Kunert-Keil et al., 2006). Several studies have demonstrated that TRPM7 is necessary for cell survival while recent findings showed that TRPM7 activation leads to cell death. NMDA channels have been shown to play a significant role in glutamate toxicity and cell death associated with ischemia (Choi, 1992; Lipton, 1999). Yet, antiexcitotoxic therapy, using NMDA channel antagonists, failed in clinical trials (Davis et al., 1997; Lees et al., 2000; Morris et al., 1999). Therefore, Tymianski and colleagues tried to find another neurotoxic process, in addition to glutamate excitotoxicity, which causes neuronal cell death (Aarts et al., 2003). They exposed cultured cortical neurons to oxygen-glucose deprivation (OGD), which mimics some conditions of brain ischemia in vitro. Prolonged OGD was found to elicit reactive oxygen/nitrogen species (ROS) production which causes a cation influx, mediated by TRPM7. The channel activation was lethal to the cells, causing neuronal cell death. Blocking the current or suppressing TRPM7 expression with siRNA prevented Ca2+ entry, ROS production and cell death. However, the siRNA reduced not only TRPM7 expression levels but also the expression level of TRPM2 (see above). Thus, although the characteristics of the OGD-induced-current (IOGD) are similar to the TRPM7 current and not to the TRPM2 current, it is still possible that TRPM2 takes part in this process. The authors suggest that there is a positive feedback loop between Ca2+ and ROS, in which Ca2+ stimulates ROS leading to elevated Ca2+ influx through the TRPM7 channel. The Ca2+ and ROS overloads are toxic to the cell (see Fig 1). Since TRPM7 is also permeable to toxic divalent metal ions (Monteilh-Zoller et al., 2003), the authors offer another possibility, in which influx of toxic divalent ions such as Zn2+ and Ni2+ through TRPM7 mediates cell death. Since cell death depends on extracellular Ca2+ concentrations, this possibility is less favorable.
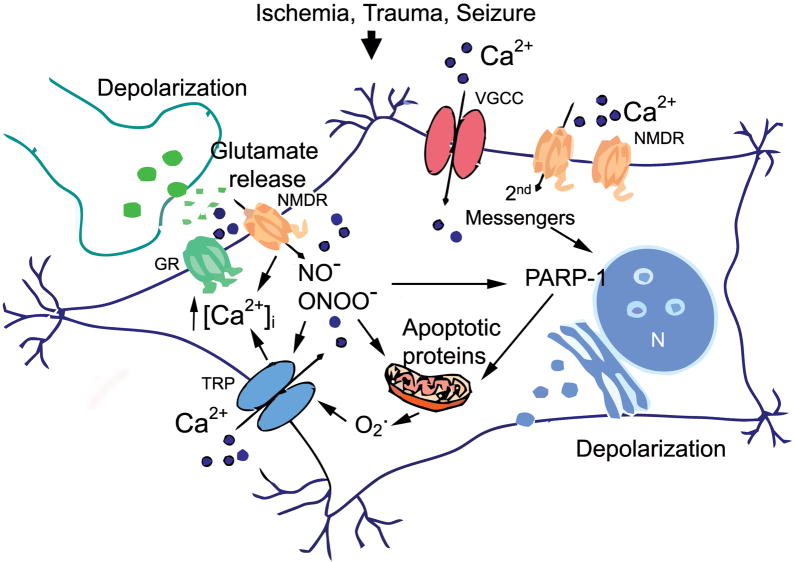
Figure 1. Excitotoxicity initiates acute neurodegeneration.
Over-activation of glutamate receptors (GR) in an excitatory synapse leads to Ca2+ influx (dark circles., Ca2+) in the post synaptic neuron, via N-methyl-D-aspartate receptors (NMDR), voltage-gated Ca2+ channels (VGC) and non-specific cation conductances via TRP channels (TRP). Ca2+ entering, in particular via NMDARs activates neurotoxic signaling cascades. During acute neuronal injury, excess nitric oxide can result in the formation of peroxynitrite, a toxic free radical that causes direct damage to cellular structures, activates further cation conductances via TRPM7 and TRPM2 channels (TRP) and promotes the formation and release of pro-apoptotic proteins [PARP-1 poly(ADP-ribose) polymerase in the nucleus,(N) ]. NMDA receptor activation during neuronal injury results in Ca2+ entry that stimulates production of nitric oxide (NO−) and release of superoxide (O2−) from mitochondria. NO and O2− then combine to form the highly reactive species peroxynitrite (ONOO−). These free radicals in turn activate TRPM7 and/or heteromeric TRPM7/TRPM2 channels resulting in further Ca2+ influx [Ca2+] and production of oxygen and nitrogen free radicals. In addition, release of ADPribose from injured mitochondria, activate TRPM2 subunits via the C-terminal ADP-ribosylase domain of TRPM2. The figure was adapted from (Aarts and Tymianski, 2005).
Recently, TRPM7 was shown to be involved in ischemic cell death in vivo (Sun et al., 2009). Tymianski and colleagues suppressed TRPM7 expression in adult rat hippocampal neurons by viral infection of specific shRNA. TRPM7 suppression resulted in decreased and delayed neuronal death induced by transient global cereberal ischemia, together with preservation of mice performance in hippocampal-dependent tasks. The authors suggest that reduction in extracellular Ca2+ and Mg2+ ions following ischemia promotes TRPM7 hyper activation, leading to Ca2+ overload.
In summary, although TRPM7 is involved in neuronal cell death, the underlying mechanism is not entirely clear but does involves an increase in intracellular Ca2+, due to opening of the Ca2+ permeable TRPM7 channel.
2.3 Transient Receptor Potential Canonical (TRPC) channels
The mammalian TRPC subfamily was discovered because of a substantial (~40%) amino acid sequence homology with the Drosophila TRP and TRPL channels (Birnbaumer et al., 1996). TRPC channel are Ca2+ permeable nonselective cation channels. The channels are expressed in many tissues including the brain. This subfamily is divided into two groups: i. TRPC1, TRPC4 and TRPC5 and ii. TRPC3, TRPC6 and TRPC7.
Pioneering biochemical (Devary et al., 1987) and genetic (Bloomquist et al., 1988) studies in Drosophila revealed that activation of TRPC channels is mediated by phospholipase C (PLC). The mammalian TRPC group ii can be activated directly by exogenous application of diacylglycerol (DAG), the product of PIP2 hydrolysis by PLC (Berridge, 1993). In addition, TRPC1, TRPC3, TRPC4 were shown to open in response to intracellular Ca2+ store depletion ( reviewed in (Abramowitz & Birnbaumer, 2009)). The Drosophila light activated TRP and TRPL can be also activated by poly unsaturated fatty acids (PUFAs) in the dark, in vivo (Chyb et al., 1999).
One of the prominent features of the Drosophila TRP and TRPL channel activation is the absolute dependence on light dependent activated PLC, under physiological conditions (Bloomquist et al., 1988; Devary et al., 1987). The first study on TRP channel activation by metabolic stress was reported for the Drosophila TRP and TRPL channels. Metabolic stress, induced by anoxia in vivo, reversibly activates TRP and TRPL channels continuously ((Agam et al., 2000), Fig. 2A). A continuous uncontrolled and maximal activation of these channels most likely leads to photoreceptor cell death due to Ca2+ overload (Agam et al., 2004). This conclusion was strongly supported by a mutation in the Drosophila TRP channel (trpP365) which makes the channel constitutively active in a similar manner to anoxia, leading to severe degeneration of photoreceptor cells in vivo ((Yoon et al., 2000), Fig. 2B) and the partial rescue of the degeneration by over-expression of the Na+-Ca2+ exchanger (Wang et al., 2005). Although metabolic stress was not suggested as the physiological gating mechanism of the channels, their extreme sensitivity to hypoxic conditions together with high Ca2+ permeability make the cells vulnerable to metabolic stress, which might develop under pathologic conditions, again showing the crucial role of TRP Ca2+ permeability with respect to cellular effects. Similar sensitivity to metabolic stress characterizes several mammalian TRPC channels (see TRPC3 and TRPC4 below).
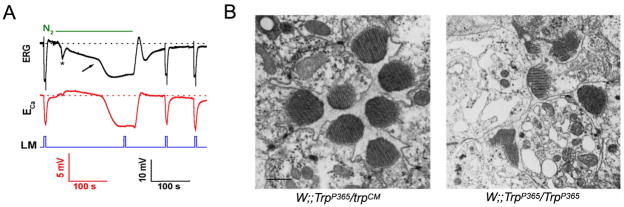
Figure 2.
A. Anoxia (N2) activated the TRP and TRPL channels in wild type (WT) Drosophila photoreceptor
In vivo extracellular voltage change (electroretinogram, ERG, top traces) and potentiometric measurements of Ca2+ influx with Ca2+ -selective microelectrode (ECa, bottom traces) in response to orange lights and anoxia in WT Drosophila are shown. Light pulses (LM) elicited voltage responses to light and Ca2+ influx before and after application of nitrogen (N2). However, there is no response to light during the maximal electrical response to anoxia because all TRP and TRPL channels are maximally activated. The calibration for the ERG records is indicated (bottom right), and the calibration for the potentiometric measurements with the Ca2+- selective microelectrode is also indicated (bottom left). The arrow indicates the activation of the TRP and TRPL channels. The figure was adapted from (Agam et al., 2000).
B. Retial degeneration of constitutively active Drosophila TRP channel in the trpP365 mutant fly Electron micrographs of transverse sections through the Drosophila ommatidial layer of TrpP365/trpCM (with only one copy of the mutated channel, left panel) and TrpP365/TrpP365 (with two copies of the mutated channel, right panel). The retina of TrpP365/TrpP365 mutant shows severe ommatidia degeneration: Some photoreceptors are missing, and some stain darkly. Scale bar, 1 μm. the figure was adapted from (Yoon et al., 2000).
TRPC1
Overexpression of TRPC1 (see below) in IEC-6 intestinal epithelial cells increased their susceptibility to apoptosis, induced by TNF-α together with cyclohexamide or staurosporine (Marasa et al., 2006). siRNA treatment targeting TRPC1 prevented the increase in susceptibility to apoptosis. The authors reported an inverse-correlation between NFκB activation and TRPC1 expression, therefore suggesting that Ca2+ influx through the TRPC1 channel inhibits NFκB activity, which then sensitizes the cells to apoptosis. In a follow-up study, Marasa and colleagues found that increased expression of TRPC1 facilitates protein phosphatase 2A activity, which results in NFκB inactivation and susceptibility to apoptosis (Marasa et al., 2008).
It is important to mention that the link between TRPC1 and apoptosis does not seem to be a general mechanism, as TRPC1 was also found to have both a neuroprotective effect (Bollimuntha et al., 2005; Bollimuntha et al., 2006; Selvaraj et al., 2009) and anti-apoptotic effect in keratinocytes isolated from Darier’s disease patients (Pani et al., 2006).
TRPC3
Cardiomyocytes, over-expressing TRPC3 were isolated from mice and showed a higher level of apoptosis in response to ischemia-reperfusion treatment, relative to cardiomyocytes isolated from wild type mice (Shan et al., 2008).
TRPC4
The TRPC4 channel (as well as TRPC3) is activated by oxidative stress (Balzer et al., 1999; Poteser et al., 2006). The channel’s opening by oxidative stress depends on PLC activation and causes depolarization via Na+ influx. However, so far, activation of TRPC4 by oxidative stress has not been related to cell death.
TRPC7
Angiotensin II induces apoptosis in myocardiocytes, which is initiated by elevation of intracellular Ca2+. Satoh and colleges over-expressed the TRPC7 channel in HEK293 cells and in cultured cardiomyocytes (Satoh et al., 2007). TRPC7-transfected HEK293 cells and cardiomyocytes showed increased intracellular Ca2+ and increased basal and angiotensin II-induced-apoptosis. The increased angiotensin II-induced-apoptosis was inhibited using the non-specific Ca2+ channel inhibitor SK&F96365, the calcineurin inhibitor FK506 or the angiotensin II Type 1 receptor blocker, CV-11974. Calcineurin has been shown to participate in apoptosis via caspase 3 activation and cytochrome c release (see review (Ermak & Davies, 2002). These results led the authors to conclude that activation of the TRPC7 channel by the angiotensin II Type 1 receptor induces elevation of intracellular Ca2+ leading to apoptosis, apparently via a calcineurin-dependent pathway.
In summary, expression of several types of TRP channels makes cells vulnerable to cell death because of their Ca2+ permeability combined with oxidative/metabolic stress activation, which is an unphysiological activation of the channels. The role of PLC in this pathological activation of the TRPC channels requires further investigations. Since a considerable fraction of TRPC channels reveal organization in multimolecular signaling complexes and signal induced translocation, which modulate cellular permeability to Ca2+ (see Introduction), the involvement of these TRP channel properties in cell death needs to be investigated in the future.
3. Proliferation and Differentiation
Intracellular Ca2+ has been established as one of the key regulators of cell proliferation and differentiation in many cell types (reviewed in (Berridge, 1995; Berridge et al., 1998; Berridge et al., 2000) see Fig. 3). Among others, one significant function of Ca2+ in controlling cell proliferation is activation of transcription factors (such as NFAT, NFκB or CREB, Fig. 3). In proliferation, the calcium signaling is required both for stimulating quiescent cells to enter the cell cycle, and for the transition between the different phases of the cell cycle. Release of Ca2+ from intracellular stores and activation of store operated channels (SOCs), are the main components responsible for the rise in cytosolic Ca2+ during proliferation and differentiation, in many cell types (Fig. 3). In recent years, Orai and several TRPC channels were established as SOCs (for review see (Cahalan, 2009)). Therefore, these channels may participate in differentiation and proliferation processes.
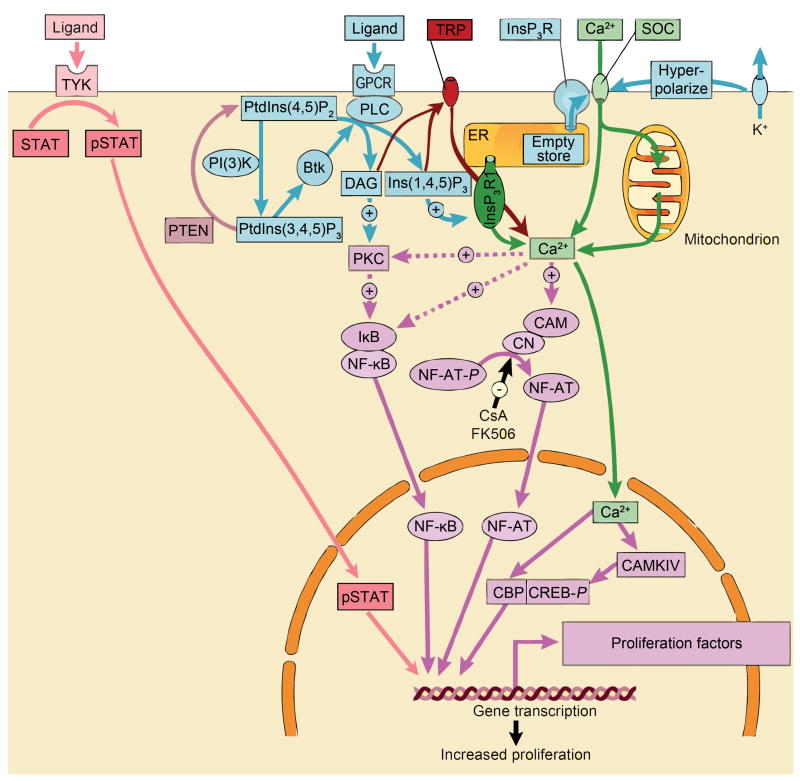
Figure 3. The critical roles of signal-induced increase of cellular Ca2+ in proliferation.
Ligand binds to the G protein coupled receptor (GPCR) to recruit phospholipase C (PLC) which generate both diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (Ins(1,4,5)P3). The production of Ins(1,4,5)P3 is maintained by the phosphatidylinositol-3-OH kinase (PI(3)K) pathway, which generates phosphatidylinositol- 3,4,5-trisphosphate (PtdIns(3,4,5)P3). This stimulates the non-receptor tyrosine kinase Btk which, in turn, phosphorylates and activates PLC. Ins(1,4,5)P3 releases Ca2+ from the endoplasmic reticulum (ER) through the Ins(1,4,5)P3 receptor (InsP3R1). Emptying of this store activates store-operated channels (SOCs). The latter are kept open by potassium channels, which hyperpolarize the membrane, and by mitochondria, which reduce the negative feedback effect of Ca2+ on the SOCs. PLC also activates TRP channels (TRP) via production of DAG and InsP3R further enhancing cellular Ca2+ elevation. Ca2+ initiates the proliferative response by stimulating various transcription factors such as NF-κB, NF-AT and CREB. The stimulatory action of Ca2+ on the calmodulin (CAM)–calcineurin (CN) complex that dephosphorylates NF-AT is inhibited by the immunosuppressants cyclosporin A (CsA) and FK506. (PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphosphate; PKC, protein kinase C; IKB, inhibitor of NF-κB; P, phosphate). Ligand binding to receptor tyrosine kinase (TYK) facilitates phosphorylation of STAT (pSTAT), which translocates to the nucleus and induces expression of the transcription factor c-jun.
The figure was modified from (Berridge et al., 2000)
Some of the studies presented below show the involvement of TRP channels in cell proliferation under pathological conditions rather than physiological conditions. Increased levels of TRP channels have been reported in many proliferating tissues under pathological conditions and the involvement of TRP channels was demonstrated in several studies utilizing RNAi techniques. These studies, although not reflecting normal tissue function, can enlighten the mechanism involving cell proliferation.
3.1 Transient Receptor Potential Canonical 1 (TRPC1)
TRPC1 is a nonselective cation channel, which shows either linear or outward rectifying I-V curves, depending on the channel’s subunit composition. TRPC1 is ubiquitously expressed in a variety of tissues such as the brain, heart, testis, ovaries, smooth muscle, endothelium, salivary glands and liver (reviewed in (Rychkov and Barritt, 2007)). TRPC1 was the first channel discovered in the search for mammalian homologs of the Drosophila TRP channel. The full length gene was cloned by two independent groups, both searching for homologous trp sequences in the EST (Expressed Sequence Tag) database (Wes et al., 1995; Zhu et al., 1995). The gene shows ~40% identity to the Drosophila trp gene.
Activation of TRPC1 was demonstrated by either Ca2+ store depletion, or independently by G protein coupled receptor (GPCR), tyrosine kinase coupled receptor and mechanical stretch (reviewed in (Ramsey et al., 2006). Endogenous TRPC1 was shown to be a component or regulator of SOC in a variety of cell types, apparently in a protein-protein interaction process via assembly with inositol trisphosphate receptor (IP3R), Stromal Interaction Molecule 1 (STIM1), the protein that couples store depletion to channel activation, or Orai1 channels (reviewed in (Ambudkar et al., 2007)).
TRPC1 is important both for the differentiation process and for proliferation of differentiated cells in several different tissues. Growing of C2C12 myoblasts cells under differentiating conditions induces elevation in the expression of TRPC1 after 24 hours, followed by decreased expression when the differentiation process progresses (Formigli et al., 2009). Increased mRNA and protein expression levels of TRPC1 and TRPC3 were also demonstrated when differentiation was induced in H19-7 hippocampal cells, which was followed by increased store operated calcium entry (SOCE) (Wu et al., 2004). Cells treated with siRNA targeting TRPC1 and TRPC3 showed inhibition in both the SOCE and differentiation. Similar results were shown in human gingival keratinocytes (Cai et al., 2006) and in the HaCaT human keratinocyte cell line, in which TRPC4 involvement was also demonstrated (Beck et al., 2008). In this respect it is relevant to mention that TRPC1 expression was found to be increased in keratinocyte from Darier’s disease patients, together with enhanced proliferation (Pani et al., 2006).
Differentiated cells usually proliferate following treatment with growth factors. Fiorio Pla and colleagues investigated the involvement of TRPC1 in bFGF-mediated proliferation of embryonic rat neural stem cells (NSC, (Fiorio et al., 2005)). They found that TRPC1 and FGF receptor 1 co-localize in NSC-derived proliferating progeny. Antisense knockdown of TRPC1 transcripts and protein led to decreased bFGF-mediated Ca2+ influx and proliferation of NSC progeny, demonstrating TRPC1 involvement in NSC proliferation. Similar results were observed in the osteoblasts cell line, in which platelet-derived growth factor induced proliferation was suppressed following treatment with TRPC1-siRNA (Abed et al., 2009). Since growth factors induce TRPC translocation, it would be interesting to examine in this preparation whether TRPC1 translocation is involved in the differentiation process.
Other studies were performed on cultured pulmonary artery smooth muscle cells (PASMC). TRPC1 expression was up-regulated in proliferative cells compared to growth-arrested cells (Golovina et al., 2001), and inhibition of TRPC1 expression with antisense oligonucleotide suppressed the store operated current and reduced cell growth (Sweeney et al., 2002). Proliferation of PASMC involved other TRPC members such as TRPC6 channel (see below) and TRPC4 channels (Zhang et al., 2004). Long term treatment with low level ATP increased CREB phosphorylation, followed by increased TRPC4 expression, enhanced capacitative Ca2+ entry (CCE) and PASMC proliferation. TRPC4-specific siRNA attenuated the ATP-induced PASMC proliferation, and overexpression of a nonphosphorylatable CREB mutant prevented both the increased TRPC4 expression and PASMC proliferation. The mechanism suggested by the authors is that low levels of ATP activate a P2 receptor which then activates PKA and PKG to phosphorylate CREB. pCREB stimulates TRPC4 expression, which results in increased CCE, mediating a positive feedback loop on CREB phosphorylation and TRPC4 expression, which ultimately leads to increased PASMC proliferation (Zhang et al., 2004).
Group one of TRPC channels includes, in addition to TRPC1, also TRPC4 (see section 2). Telencephalic human neuroepithelia (hNE) and postmitotic neurons (PMNs) generated from embryonic stem cells display robust Ca2+ transients. These Ca2+ transients in PMNs exhibited sensitivity to shRNA-mediated knockdown of the TRPC1 channel. Functionally, inhibition of Ca2+ transients in dividing hNE cells led to a significant reduction in proliferation, where as either pharmacological inhibition or shRNA-mediated knockdown of TRPC1 and TRPC4 channels, significantly reduced neuritis extension in PMNs. This study thus shows that TRPC1 and TRPC4 are parts of a mechanism for controlling Ca2+ transients in human neurons (Weick et al., 2009).
In general, the emerging mechanisms suggest that TRPC1 is responsible for the SOC current, and that increased expression of TRPC1 is required for cell proliferation/differentiation. The possible involvement of TRPC1 translocation has not been investigated. However, the role of protein-protein interactions was recently study by Pani and colleagues. They showed that the magnitude of store operated Ca2+ entry (SOCE) is regulated by STIM1 and caveolin-1 (Cav1) expression (Pani et al., 2009). These authors suggest that the exact targeting of TRPC1 to the plasma membrane (PM)-ER domains is important for TRPC1 interaction with STIM1 and for its functioning as SOC. Interaction of TRPC1 and Cav1 localizes the channel to the PM-ER domains, where it can interact with STIM1. STIM1 then dissociates TRPC1 from its interaction with Cav1 and activates it. Indeed, the author found that over-expression of Cav1 prevented TRPC1-induced proliferation and over-expression of STIM1 enhanced the proliferation.
In summary, protein-protein interaction of TRPC1 and other signaling proteins is involved in proliferation. Since growth factor induced proliferation was suppressed following treatment with TRPC1-siRNA, and growth factors are also involved in TRPC translocation, the later mechanism may be involved in differentiation and proliferation. The multiple activation mechanisms of TRPC1 and its involvement in diverse cellular functions suggest that the special properties of TRPC channels (see Introduction) are probably required for its diverse cellular functions. The seemingly contradictory functions of TRPC1 may be explained by its promiscuous nature. TRPC1 has a wide expression pattern in many tissues and has the ability to interact with many signaling proteins and forms heteromultimers with several other TRP channels. These properties of TRPC1 may underlie the multifunctionality of this channel in different cells and tissues.
3.2 Transient Receptor Potential Canonical 6 (TRPC6)
TRPC6 channel is a non-selective cationic channel with a dual rectifying current-voltage relationship. The channel is expressed mainly in smooth muscle and heart cells but also in the brain. TRPC6 was first cloned from mouse brain (Boulay et al., 1997). It shares 70% identity with TRPC3 and TRPC7 channels, and these three channels are considered as a group within the TRPC subfamily.
TRPC6 is diglycosylated on asparagine residues in the first and second extracellular loops of the transmembrane domain. The glycosylation pattern plays a role in regulating basal channel activity (Dietrich et al., 2003). TRPC6 is activated by G protein coupled receptor and PLC, although it can also be activated directly by DAG (Hofmann et al., 1999).
In a study similar to the study on TRPC1, Yu and coworkers found that the growth factor PDGF mediates the proliferation of PASMC, and induces an increase in TRPC6 expression and in the SOC current (ISOC, (Yu et al., 2003)). Reducing TRPC6 expression using antisense oligonucleotides, results in reduced ISOC and PDGF-mediated PASMC proliferation. Yu et al. further investigated the mechanism of PDGF induced TRPC6 up-regulation in proliferating PASMC, and found that the expression of TRPC6 was also increased in PASMC overexpressing the transcription factor c-jun. In addition, PDGF-induced-TRPC6 upregulation was reduced in PASMC treated with specific antisense oligonucleotides targeting c-jun. The authors suggested the following mechanism for the PDGF-induced-increased expression of TRPC6: Upon the binding of PDGF to its receptor, STAT3, a protein downstream to PDGF-receptor signaling, is activated and induces an increase in c-jun expression, which is followed by an increase in TRPC6 expression. Increased TRPC6 expression increases ISOC, which induces proliferation. An additional support for this mechanism came from their later study, showing that TRPC6 expression correlates with cell proliferation of PASMCs (Yu et al., 2004). The proliferation of PASMCs from patients with idiopathic pulmonary arterial hypertension (IPAH) was reduced following siRNA-mediated knockdown of TRPC6 expression, suggesting that the increased proliferation of PASMC in IPAH patients involves up-regulation in TRPC6 expression.
El Boustany and colleagues also found a correlation between TRPC6 expression level and cell proliferation in the human hepatoma cell lines Huh-7 and HepG2 (El et al., 2008). Overexpression of TRPC6 in Huh-7 cells resulted in an increase in the cell proliferation rate and knockdown of TRPC6 expression had the opposite effect. The magnitude of SOCE also correlates with TRPC6 expression levels, although SOCE amplitude was also attenuated when STIM1 and Orai1 levels were lowered, suggesting that these proteins work together, possibly via protein-protein interactions, characteristic of TRPC channels.
Apart from a correlation between TRPC6 expression and proliferation, a link was found between TRPC6 and the transcription factor Nuclear Factor of Activated T cells (NFAT). In search for the role of α1-adrenergic receptor (α1-AR) signaling in proliferation of prostate cancer cell, Thebault and colleagues stimulated the α1-AR in primary human prostate cancer epithelial cells by phenylephrine. α1-AR stimulation resulted in intracellular Ca2+ oscillations, elevation in TRPC6 and CDK4 (a protein important for cell cycle) expression levels, NFAT activation and reduction in p27 (a cell cycle inhibitor) expression, together with increased cell proliferation (Thebault et al., 2006). Both the Ca2+ oscillation and cell proliferation were suppressed when using antisense nucleotide specific to TRPC6. Similar results were demonstrated in primary cells and cell lines derived from glioblastoma multiforme (Chigurupati et al., 2010). In these cells, hypoxia treatment induces aggressive growth. Hypoxic treatment also activated the Notch signaling, and increased TRPC6 expression, increased intracellular Ca2+ and NFAT activation. The increased TRPC6 expression required Notch signaling, and lead to gliomas growth, while reduction in TRPC6 expression, using specific siRNA, prevented the aggressive glioma growth and invasion, Ca2+ elevation and NFAT activation. The transcription factor NFAT is regulated by Ca2+ and is known to have a role in cellular proliferation. Interestingly, the promoter of the trpC6 gene contains two conserved NFAT sites, which are required for activation of the promoter in response to calcineurin -NFAT signaling. This data correlates with the results reported by Kuwahara and colleagues, who showed that TRPC6 expression is increased in mice expressing activated calcineurin (NFAT activator), which display heart hypertrophy (Kuwahara et al., 2006). Kuwahara et al. also showed that over-expression of TRPC6 in transgenic mice induces NFAT activation, suggesting that there is reciprocal regulation between TRPC6 and NFAT in pathological conditions.
The above studies suggest that TRPC6 might be a target for therapeutic treatment. Further indications that TRPC6 is a candidate for anti cancer therapy were provided by two recent studies (Cai et al., 2009; Ge et al., 2009). These investigators showed that inhibition of TRPC6 activity by the dominant negative isoform, DNC6, inhibited both gastric cancer cells proliferation and VEGF induced angiogenesis (Cai et al., 2009; Ge et al., 2009). Inhibition of cell proliferation was mediated by cell cycle arrest, inhibiting the G2/M phase transition. Moreover, human gastric cells, AGS, which were infected with the dominant negative isoform, DNC6, formed smaller tumors when injected to nude mice, compared with wt AGS cells (Cai et al., 2009). This result demonstrates that TRPC6 is involved in gastric tumor growth, in vivo.
In summary, studies on TRPC6 revealed many cases in which the special properties of TRPC channels are involved in proliferation. This is especially true for the regulation of TRPC6 expression levels by transcription factors, while the modulation in TRPC6 expression affect cellular Ca2+ and induces proliferation. As illustrated above transcriptional regulation of proteins is often under hormonal or growth factors control. This is of special interest in the case of TRP channels involved in cellular proliferation under pathological conditions, which are hormone dependent, such as TRPM8 expression in prostate cancer (see below).
3.3 Transient Receptor Potential Melastatin 7 (TRPM7)
Under physiological conditions, the role of the TRPM7 channel is related to cell survival and proliferation, which is in contrast to the findings mentioned above, showing that TRPM7 mediates cell death in pathological conditions. Global TRPM7 knockout is lethal at early embryonic stages of mice (Jin et al., 2008). Similarly, targeted deletion of TRPM7 in DT-40 B cells is also lethal (Nadler et al., 2001). These findings were the first hint of the requirement for TRPM7 during cell proliferation. Inducible conditional deletion of TRPM7, using the tamoxifen-controlled Cre recombinase, also induces growth arrest and death of the cells within 48–72 hours (Nadler et al., 2001), although addition of high Mg2+ concentrations to the extracellular solution rescues the cells (Schmitz et al., 2003). These results suggest that (at least in this cell line) TRPM7 has a fundamental role in cellular function of cell proliferation. It might also be involved in regulation of cellular Mg2+ homeostasis, or supply the need for Mg2+ during cell replication. In this respect, it is relevant to mention that TRPM6, a close relative of TRPM7, also has a kinase domain. TRPM6 is essential for Mg2+ homeostasis and a mutation in TRPM6 causes familial hypomagnesemia (Chubanov et al., 2005). Mice lacking TRPM6 usually die at embryonic day 12.5, and almost never survived to weaning (Walder et al., 2009). The majority of mice that are born have neural tube defects.
Additional studies support a more direct involvement of TRPM7 in cell proliferation. Angiotensin II induces cell growth in vascular smooth muscle cells (VSMC), together with elevation in TRPM7 expression and intracellular Mg2+ concentrations. The angiotensin II-induced-elevation in intracellular Mg2+ concentration, as well as the cell growth, was suppressed in cells treated with siRNA targeted specifically to TRPM7 (He et al., 2005). A decrease in cell proliferation was also shown in human osteoblast-like cells (Abed et al., 2009) and retinoblastoma (RB) cells (Hanano et al., 2004) treated with TRPM7-siRNA. Cells from a variety of tissues demonstrated Ca2+ requirement for cell cycle progress, especially for the G1 to S phase transition (Takuwa et al., 1995; Takuwa and Takuwa, 1996). BrdU incorporation assay tested the above notion in RB cells treated with TRPM7-siRNA. Indeed, an inhibition of the G1 to S phase transition in the cell cycle was demonstrated (Hanano et al., 2004). Another alternative explanation is that Mg2+ decreases the abundance of the cyclin inhibitor p27Kip1. Thus, a decrease in intracellular Mg2+ concentration would increase the abundance of the inhibitor and could arrest the cells at G1. TRPM7 dependence for correct proliferation and differentiation was also shown in zebrafish, in which mutations in TRPM7 cause severe growth retardation and general alterations in skeletal muscle development (Elizondo et al., 2005).
In summary, based on the above observations, the current notion is that the TRPM7 channel is required for cell cycle in many cell lines but it is not required for cell maintenance. This view was recently supported by localized knock down of TRPM7, which did not cause cell death in vivo under physiological condition (Sun et al., 2009). The role of TRPM7 in controlling proliferation seems to involve not only its Ca2+ permeability, like for other TRP channels, but also its Mg2+ permeability.
3.4 Transient Receptor Potential Melastatin 8 (TRPM8)
The TRPM8 cDNA was initially cloned in a screen for mRNA up-regulation in prostate cancer (Tsavaler et al., 2001). Later on, the TRPM8 cDNA was isolated by expression cloning using mRNA from trigeminal neurons (McKemy et al., 2002; Peier et al., 2002). Then, TRPM8 was identified as an outwardly rectifying Ca2+ permeable non selective cation channel, which is activated by cold temperatures below 28 °C (McKemy et al., 2002; Peier et al., 2002). The localization of this channel in cold responsive small diameter neurons of dorsal root ganglia and trigeminal ganglia is consistent with a role in cold sensation. However, the function and activation mechanism of TRPM8 in other tissues is largely unknown. TRPM8 can be activated by natural compounds such as eucalyptol and menthol as well as by the synthetic super cooling agent icilin (EC50 in the nM range,(McKemy et al., 2002). All TRPM8 agonists induce a cooling sensation, suggesting that TRPM8 is a cold receptor. Urea compounds and capsazerpin, which inhibit TRPV1, are also TRPM8 antagonists.
TRPM8 mRNA is predominantly expressed in malignant cells and it was mainly studied in prostate cancer (Bidaux et al., 2005; Fuessel et al., 2003; Tsavaler et al., 2001). TRPM8 transcript was found to be significantly up-regulated in tissue samples of prostate cancer patients ((Fuessel et al., 2003), for a review see (Bodding, 2007)). TRPM8 expression is under androgen regulation and loss of TRPM8 mRNA expression was associated with transition to an androgen independent stage of prostate cancer. The analysis of the TRPM8 gene resulted in 10 putative androgen responsive elements, one in the promoter region and others in introns of the gene (Bidaux et al., 2005; Zhang and Barritt, 2004). Binding of the testosterone androgen receptor complex to these androgen responsive elements might initiate TRPM8 gene transcription. TRPM8 is mainly expressed in androgen-dependent, apical secretory epithelial cells of the prostate. Although TRPM8 is a Ca2+ permeable channel involved in tumor progression, the underlying mechanism in tumor progression is still unknown.
3.5 Transient Receptor Potential Vanilloid 4 (TRPV4)
TRPV4 is a non selective Ca2+ channel, with an outward rectification, probably due to a Ca2+ open channel block, since removal of Ca2+ from the extracellular solution causes linearization of the I-V curve (Voets et al., 2002). The channel is expressed in the heart, brain, endothelium, liver, kidney, placenta, lung, trachea and salivary gland (see review (Plant and Strotmann, 2007)). TRPV4 was found in a screen aimed at finding homolog proteins for the known TRPV channels, and was first given several names: VR-OAC (vanilloid receptor-related osmotically activated channel) (Liedtke et al., 2000), TRP12 (Wissenbach et al., 2000), Osm-9-like TRP channel 4 (OTRPC4, (Strotmann et al., 2000)) and vanilloid receptor-like channel 2 (VRL-2, (Delany et al., 2001)). Although TRPV4 was initially discovered as an osmotically activated channel, the channel is also activated by warm temperatures (>25° C, (Chung et al., 2003; Guler et al., 2002; Watanabe et al., 2002b)), as well as endocannabinoids (Watanabe et al., 2003) and phorbol ester derivatives (Watanabe et al., 2002a).
Matsuyama and colleagues investigated bone homeostasis in TRPV4 knock-out mice, and found that although bone development was not affected in TRPV4−/− mice, the bone mass was higher in 12 weeks old TRPV4−/− mice compared to control mice (Masuyama et al., 2008). The increased bone volume results from decrease in osteoclast number and decreased bone resorption. They showed that mRNA levels of TRPV4 increases in correlation with osteoclast maturation, and the localization of TRPV4 is restricted to the basolateral membrane, indicating that TRPV4 is not involved in transcellular Ca2+ transport during bone resorption. During osteoclast differentiation, the binding of the osteoclastogenic factor RANKL (receptor activator of NF-κB ligand) to its receptor induces Ca2+ signaling, which leads to NFATc1 activation. TRPV4 activation induces activation of NFATc1 even when RANKL is removed, implying that TRPV4 enhances the differentiation of mature osteoclast. The author investigated TRPV4 involvement in osteoclast differentiation and found that TRPV4 is not responsible for the Ca2+ oscillation at the beginning of osteoclast differentiation but rather, is responsible for the sustained Ca2+ influx necessary for terminal differentiation of the osteoclast (Masuyama et al., 2008).
Another study suggests the involvement of TRPV4 in chondrocytes differentiation. Muramatsu and colleagues found that following induction of differentiation, TRPV4 induces increased activation of the SOX9 promoter (Muramatsu et al., 2007). SOX9 is a transcription factor that has an essential role in differentiation of chondrocytes. Extracellular Ca2+ is required for TRPV4-induced elevation in SOX9 levels, and calmodulin might be involved as well, as calmodulin inhibitor attenuates the TRPV4 induced increase in SOX9 levels. Application of a TRPV4 agonist following differentiation induction also increases the expression level of COL2A1 and aggrecan, two differentiation markers. These results indicate that TRPV4 might participate in chondrocytes differentiation. In summary, the role of TRPV4 in proliferation of specific cells arises from its Ca2+ permeability, which regulates transcription factor levels.
3.6 Transient Receptor Potential Vanilloid 6 (TRPV6)
TRPV6, as well as TRPV5, form unique channels among the TRP superfamilies, as both channels are highly selective to Ca2+ (Hoenderop et al., 2001; Hoenderop and Bindels, 2005). The Ca2+ selectivity is achieved due to an aspartate residue in the pore region (Nilius et al., 2001). The channel demonstrates inward rectification (Voets et al., 2001; Voets et al., 2003) and it is expressed in the intestine, kidney, pancreas, placenta and salivary gland (the expression pattern differs between different organisms (For details see a recent review (Wissenbach and Niemeyer, 2007)). In a screen to find a protein responsible for Ca2+ influx in the duodenum, Peng et al. cloned the calcium transport protein (CaT1, (Peng et al., 1999)) which shows similarity to the TRP channels superfamily and later was named TRPV6. the channel was also named CaT-like (CaT-L, (Wissenbach et al., 2001)), and ECaC2 (Barley et al., 2001). The channel is constitutively active in heterologous systems, but at hyperpolarizing potentials it can be inactivated by extracellular calcium (Peng et al., 1999; Wissenbach et al., 2001). The channel has binding sites for calmodulin, and conflicting results show that calmodulin either inactivates the channel or has a positive effect on the activation (Derler et al., 2006; Lambers et al., 2004). RGS2, which belongs to the RGS family (involved in termination of GPCR signaling) interacts with the channel and inhibits its activation (Schoeber et al., 2006).
TRPV6 was found to have a role in keratinocytes differentiation (Lehen’kyi et al., 2007a). Keratinocytes differentiation can be induced by a sudden increase in high extracellular Ca2+ concentration (Ca2+ switch). The Ca2+ switch is followed by increase in intracellular Ca2+ together with up-regulation in specific differentiation markers, such as IVL, transglutaminase 1 (TGM1) and cytokeratin 10 (KRT10). Lehen’kyi and colleagues found that the expression of TRPV6 was also up-regulated in differentiated keratinocytes. Keratinocytes treated with siRNA against TRPV6 do not demonstrate the elevation in intracellular Ca2+ following a Ca2+ switch. Moreover, the expression of specific-differentiation markers is decreased in TRPV6-siRNA treated cells, compared to non treated-cells in the same conditions, suggesting that TRPV6 is required for keratinocytes differentiation.
TRPV6 protein level was found to be elevated in prostate, breast, thyroid, colon, and ovarian carcinomas, relative to normal tissues (Zhuang et al., 2002). TRPV6 expression correlates with prostate tumor grade and aggressiveness (Fixemer et al., 2003; Wissenbach et al., 2001), and was found to be involved in prostate cancer proliferation rate(Lehen’kyi et al., 2007b). In a similar manner to TRPM8, TRPV6 expression is also under androgen hormonal regulation in the prostate however, this is a negative regulation. Accordingly, androgen treatment of the human Lymph Node Prostate cancer (LNCaP) cell line, which constitutively expresses TRPV6, reduces its mRNA level by 80% within one day. The hormonal regulation of TRPV6 expression includes also estrogen, since estrogens positively regulates TRPV6 transcription in breast cancer cells. Tamoxifen, an estrogen receptors blocker, is widely used in treatment of breast cancer in order to reduce the growth signals to the cells, and was found to reduce TRPV6 transcription. These observations suggest that the estrogen receptor regulates TRPV6 expression. Interestingly, the estrogen 17β-estradiol exerts a specific and direct effect on the TRPV6 channel (Irnaten et al., 2008). Thus, dual regulation of TRPV6 action in proliferation of breast cancer cells may take place: transcriptional regulation and direct estrogen regulation. Accordingly, estrogen-induced Ca2+ influx via TRPV6 may operate synergistically with estrogen to amplify channel transcription (for a review see (Gkika and Prevarskaya, 2009).
In summary, TRP channels, which belong to several subfamilies, are involved in proliferation and differentiation. One obvious common denominator of all these cellular functions is Ca2+ influx through these TRP channels, which activates transcription factors that activate proliferation and differentiation mechanism (see Fig. 3). However, the complexity of the regulations by hormones, growth factors and protein-protein interactions suggest that other properties of TRP channels including multimolecular organization and signal induced translocation may also be involved and require further investigation.
4. Transient Receptor Potential Melastatin 7 and transmitter release
Vesicle fusion and recycling during synaptic transmission is a highly coordinated process which is regulated by many proteins. Following a stimulus, the vesicles translocate to a specific membrane region, fuse with the membrane and release their content. The commonly accepted mechanism suggests that trafficking and fusion of the vesicles to the plasma membrane requires Ca2+ and the participation of the SNARE proteins. This mechanism applies to synaptic and secretory vesicles fusion and endocytosis in general. Ion channels have been proposed to participate in this process, and several ion channels are localized to the membrane of synaptic vesicles (Arispe et al., 1992; Kelly and Woodbury, 1996; Rahamimoff et al., 1988), although their actual role in neurotransmitter release has yet to be determined.
A yeast two-hybrid assay was applied to find binding partners of TRPM7. Snapsin was one of the proteins found (Krapivinsky et al., 2006). Since snapsin is a synaptic vesicle protein, the authors first confirmed that TRPM7 is localized to the synaptic vesicles and then found that TRPM7 also interacts with synapsin I and synaptotagmin. Snapsin is known to affect synaptic transmission. In order to find out if TRPM7 is also involved in regulation of vesicles fusion, the authors assayed the vesicular synaptic release. By measuring the amplitude and kinetics of excitatory post synaptic potentials (EPSP) from superior cervical ganglion (SCG) postsynaptic neurons with altered TRPM7 levels, they found a correlation between TRPM7 expression levels and EPSP amplitude, quantal size and decay time. Expression of a dominant-negative TRPM7 mutant in SCG neurons resulted in complete suppression of the postsynaptic responses, suggesting that the ion conductance properties of TRPM7, rather than the protein itself, is important for this process. Disruption of the TRPM7-snapsin binding, by injection of either TRPM7 or snapsin fragments that contain the binding site, resulted in an effect that was similar to reduction in TRPM7 expression, indicating that TRPM7 interaction with snapsin is also important for vesicles release. A reduction in TRPM7 levels seems to affect the quantal size (the amount of neurotransmitter released from a single synaptic vesicle) rather than the number of released vesicles. The authors suggest that TRPM7 may affect quantal release size either by altering the kinetics of pore fusion, or by changing the transvesicular voltage, which in turn may affect neurotransmitter release. Another mechanism proposed by the authors is that TRPM7 affects the amount of mobile transmitter contained in a vesicle. Within the vesicles, It has been suggested that positively charged neurotransmitters such as acetylcholine are immobilized in a polymeric ion exchange matrix (Rahamimoff and Fernandez, 1997). In order to release the neurotransmitters from this matrix, positively charged counterions are required, which would replace the neurotransmitter. Cholinergic vesicles contain the positively charged acetylcholine. Therefore, cations influx through the TRPM7 can replace acetylcholine (but not the negatively charged glutamate in glutamatergic synapse) and release it from the negatively charged matrix (Fig. 4).
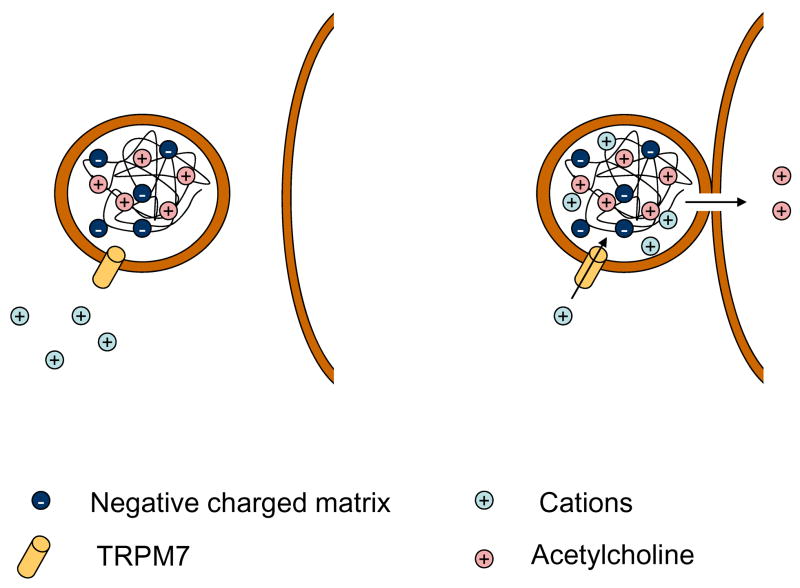
Figure 4. A model for an Ion exchange–dependent secretory response mediated via TRPM7.
A scheme of synaptic vesicle is presented
Left panel: The fixed negative charges of an ion exchange matrix (heavily tinted circles marked by “−“) are compensated by an equal number of cationic secretory products of the neurotransmitter acetylcholine. Release by ion exchange consists on replacing an existing cation (acetylcholine, medium tinted circles) by a new cation (slightly tinted circles). The movement of all ions through the matrix is determined by its ion exchange properties.
Right panel: The opening of a fusion pore triggers ion exchange
The flow of counter-ions through the TRPM7 channel, located on the vesicle membrane, and through the fusion pore, replaces the positive charged acetylcholine that can exit from the vesicle.
The figure was modified from (Rahamimoff & Fernandez, 1997)
In order to develop a more direct measure of fusion events, the authors next studied vesicles fusion in PC12 cells (Brauchi et al., 2008), which also contain acetylcholine-secreted-synaptic-like vesicles, and endogenously express TRPM7. They targeted the pH-sensitive GFP variant, pHluorin, to the lumen of the vesicles, so it could serve as a reporter of synaptic function and vesicular fusion. TRPM7 specific siRNA inhibited vesicles fusion, as did the expression of dominant negative TRPM7.
A major unanswered question is how TRPM7 is activated to allow its ion exchange function. One possibility is that PIP2 on the surface membrane induces activation during vesicle fusion. Interactions with PIP2 have been reported for a large number of TRP channels. TRPM7 was the first mammalian TRP that was reported to require PIP2 for activity. It was shown that hydrolysis of PIP2 by PLC inhibits TRPM7. TRPM7 activity was inhibited upon muscarinic receptor activation of PLC, while channel activity, after washout of the agonist, was recovered faster after application of PIP2 via the patch pipette or slowed down if PIP2 synthesis was inhibited by wortmannin (a PI4 kinase inhibitor, (Runnels et al., 2002)). An additional study reported on bradykinin activation of TRPM7 via PLC activation in a perforated patch clamp configuration, but inhibits TRPM7 in whole cell configuration if Mg2+ is not present in the pipette. As mentioned above, Intracellular Mg2+ was reported to inhibit TRPM7. Taken together the available data suggest that TRPM7 requires PIP2 for activity but the physiological context of TRPM7 regulation by depletion of PIP2 needs further investigation (reviewed in (Rohacs, 2009)). Accordingly, the activation mechanism of TRPM7 in the synaptic vesicle is still unknown.
Taken together, TRPM7 channel regulation of neurotransmitter release is an important finding, which may motivate the search for the next ion channel which participates in vesicular fusion. Since TRPM7 is expressed in almost every tissue, it is interesting to test the localization of TRPM7 in other transmitter-releasing-vesicles, to see whether this is a common mechanism. It is equally important to determine whether other TRP channels also participate in transmitter release mechanisms.
5. Future prospects
TRP channels are essential components of biological sensors that detect changes in the external and internal environment in response to a myriad of stimuli. One of the most important features of TRP channels is their Ca2+ permeability, making them crucial components in cellular calcium homeostasis. It is therefore important to direct future efforts towards elucidating the molecular and cellular mechanisms underlying a specific TRP channel participation in a specific Ca2+ dependent cellular mechanism. Importantly, several roles, which are sometimes conflicting, can be attributed to the same channel (i.e. the effects of TRPM7 on cell death versus cellular survival) suggesting that the timing, spatial expression and interactions with other proteins are of outmost importance. Moreover, expression of several TRP channels in the same cell and the possible involvement of other Ca2+ permeable channels make this investigation particularly challenging and requires application of a multidisciplinary approach. A genetic approach is particularly useful for this task in spite of the redundancy of mammalian TRP channels due to expressions of several TRP channels in a single cell. The conservation of TRP channels through evolution makes the use of C. elegans and Drosophila melanogaster useful because of the power of molecular genetics in these animal models and the minimal redundancy among various TRP channels of these preparations. Studying TRP channels in their native cellular environment is also important because the specific cellular environment and cellular expression levels seem to be important for the normal physiological function of a specific TRP channel in a specific cellular task.
The specific features of TRP channels that allow them to play important roles in diverse cellular functions were illustrated in this review. Strikingly, TRPM7 plays a role in all three cellular functions described in this review. What feature of TRPM7 makes it suitable to participate in these, sometime contradictory, functions such as cell death, cell survival and differentiation? One possible answer is that its unique composition of an ion channel together with a protein kinase allows the multi-potent activity. Future studies will have to assess such a possibility.
Abbreviations
- TRP
transient receptor potential
- ADPR
ADP ribose
- ROS
reactive oxygen/nitrogen species
- PARP
poly ADP ribose polymerase
- SOC
store operated channel
- SOCE
store operated calcium entry
- CCE
capacitative calcium entry
- TNF-α
tumor necrosis factor-α
- PLC
phopholipase C
- PIP2
phosphatidylinositol 4,5 bisphosphate
- OGD
oxygen-glucose deprivation
- DAG
diacylglycerol
- EST
Expressed Sequence Tag
- GPCR
G protein coupled receptor
- IP3R
inositol trisphosphate receptor
- STIM1
stromal interaction molecule 1
- PMN
post mitotic neurons
- bFGF
Basic Fibroblast Growth Factor
- NSC
neural stem cells
- PASMC
pulmonary artery smooth muscle cells
- CREB
cAMP response element binding protein
- NFAT
nuclear factor of activated T cells
- PDGF
platelet-derived growth factor
- α1-AR
α1-adrenergic receptor
- Cav1
caveolin 1
- RB
retinoblastoma
- EPSP
excitatory post synaptic potential
- SCG
superior cervical ganglion
- shRNA
short hairpin RNA
- VEGF
vascular endothelial growth factor
- SNARE
soluble NSF attachment protein receptors
- RGS
regulators of G protein signaling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–77. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Aarts MM, Tymianski M. TRPMs and neuronal cell death. Pflugers Arch. 2005;451:243–9. doi: 10.1007/s00424-005-1439-x. [DOI] [PubMed] [Google Scholar]
- Abed E, Labelle D, Martineau C, Loghin A, Moreau R. Expression of transient receptor potential (TRP) channels in human and murine osteoblast-like cells. Mol Membr Biol. 2009;26:146–58. doi: 10.1080/09687680802612721. [DOI] [PubMed] [Google Scholar]
- Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agam K, Frechter S, Minke B. Activation of the Drosophila TRP and TRPL channels requires both Ca2+ and protein dephosphorylation. Cell Calcium. 2004;35:87–105. doi: 10.1016/j.ceca.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Agam K, von-Campenhausen M, Levy S, Ben-Ami HC, Cook B, Kirschfeld K, et al. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J Neurosci. 2000;20:5748–55. doi: 10.1523/JNEUROSCI.20-15-05748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42:213–23. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Arispe N, Pollard HB, Rojas E. Calcium-independent K+-selective channel from chromaffin granule membranes. J Membr Biol. 1992;130:191–202. doi: 10.1007/BF00231896. [DOI] [PubMed] [Google Scholar]
- Bahner M, Frechter S, Da SN, Minke B, Paulsen R, Huber A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. doi: 10.1016/s0896-6273(02)00630-x. [DOI] [PubMed] [Google Scholar]
- Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, et al. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 2008;9:472–9. doi: 10.1038/embor.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzer M, Lintschinger B, Groschner K. Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovascular Research. 1999;42:543–9. doi: 10.1016/s0008-6363(99)00025-5. [DOI] [PubMed] [Google Scholar]
- Barley NF, Howard A, O’Callaghan D, Legon S, Walters JR. Epithelial calcium transporter expression in human duodenum. Am J Physiol Gastrointest Liver Physiol. 2001;280:G285–G290. doi: 10.1152/ajpgi.2001.280.2.G285. [DOI] [PubMed] [Google Scholar]
- Beck B, Lehen’kyi V, Roudbaraki M, Flourakis M, Charveron M, Bordat P, et al. TRPC channels determine human keratinocyte differentiation: new insight into basal cell carcinoma. Cell Calcium. 2008;43:492–505. doi: 10.1016/j.ceca.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signalling and cell proliferation. Bioessays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Lipp P. Calcium--a life and death signal. Nature. 1998;395:645–8. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–20. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Bidaux G, Roudbaraki M, Merle C, Crepin A, Delcourt P, Slomianny C, et al. Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: functional androgen receptor requirement. Endocr Relat Cancer. 2005;12:367–82. doi: 10.1677/erc.1.00969. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Zhu X, Jiang MS, Boulay G, Peyton M, Vannier B, et al. On the molecular basis and regulation of cellular capacitative calcium entry: Roles for Trp proteins. Proc Natl Acad Sci U S A. 1996;93:15195–202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–33. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Bodding M. TRP proteins and cancer. Cell Signal. 2007;19:617–24. doi: 10.1016/j.cellsig.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Bollimuntha S, Ebadi M, Singh BB. TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis. Brain Res. 2006;1099:141–9. doi: 10.1016/j.brainres.2006.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimuntha S, Singh BB, Shavali S, Sharma SK, Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. Journal of Biological Chemistry. 2005;280:2132–40. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, et al. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. Journal of Biological Chemistry. 1997;272:29672–80. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- Brauchi S, Krapivinsky G, Krapivinsky L, Clapham DE. TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proc Natl Acad Sci U S A. 2008;105:8304–8. doi: 10.1073/pnas.0800881105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD. STIMulating store-operated Ca2+ entry. Nat Cell Biol. 2009;11:669–77. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Ding X, Zhou K, Shi Y, Ge R, Ren G, et al. Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int J Cancer. 2009;125:2281–7. doi: 10.1002/ijc.24551. [DOI] [PubMed] [Google Scholar]
- Cai S, Fatherazi S, Presland RB, Belton CM, Roberts FA, Goodwin PC, et al. Evidence that TRPC1 contributes to calcium-induced differentiation of human keratinocytes. Pflugers Arch. 2006;452:43–52. doi: 10.1007/s00424-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Carini R, Autelli R, Bellomo G, Albano E. Alterations of cell volume regulation in the development of hepatocyte necrosis. Exp Cell Res. 1999;248:280–93. doi: 10.1006/excr.1999.4408. [DOI] [PubMed] [Google Scholar]
- Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. Journal of Biological Chemistry. 2004;279:7241–6. doi: 10.1074/jbc.M312042200. [DOI] [PubMed] [Google Scholar]
- Chigurupati S, Venkataraman R, Barrera D, Naganathan A, Madan M, Paul L, et al. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res. 2010;70:418–27. doi: 10.1158/0008-5472.CAN-09-2654. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–76. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Chubanov V, Gudermann T, Schlingmann KP. Essential role for TRPM6 in epithelial magnesium transport and body magnesium homeostasis. Pflugers Arch. 2005;451:228–34. doi: 10.1007/s00424-005-1470-y. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. Journal of Biological Chemistry. 2003;278:32037–46. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–9. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–7. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Davis SM, Albers GW, Diener HC, Lees KR, Norris J. Termination of Acute Stroke Studies Involving Selfotel Treatment. ASSIST Steering Committed. Lancet. 1997;349:32. doi: 10.1016/s0140-6736(05)62166-6. [DOI] [PubMed] [Google Scholar]
- Delany NS, Hurle M, Facer P, Alnadaf T, Plumpton C, Kinghorn I, et al. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol Genomics. 2001;4:165–74. doi: 10.1152/physiolgenomics.2001.4.3.165. [DOI] [PubMed] [Google Scholar]
- Derler I, Hofbauer M, Kahr H, Fritsch R, Muik M, Kepplinger K, et al. Dynamic but not constitutive association of calmodulin with rat TRPV6 channels enables fine tuning of Ca2+-dependent inactivation. J Physiol. 2006;577:31–44. doi: 10.1113/jphysiol.2006.118661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary O, Heichal O, Blumenfeld A, Cassel D, Suss E, Barash S, et al. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc Natl Acad Sci U S A. 1987;84:6939–43. doi: 10.1073/pnas.84.19.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Mederos YS, Gollasch M, Gross V, Storch U, Dubrovska G, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–9. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. Journal of Biological Chemistry. 2003;278:47842–52. doi: 10.1074/jbc.M302983200. [DOI] [PubMed] [Google Scholar]
- Du J, Xie J, Yue L. Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc Natl Acad Sci U S A. 2009;106:7239–44. doi: 10.1073/pnas.0811725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El BC, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, Capiod T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology. 2008;47:2068–77. doi: 10.1002/hep.22263. [DOI] [PubMed] [Google Scholar]
- Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, et al. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr Biol. 2005;15:667–71. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38:713–21. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Fiorio PA, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, et al. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci. 2005;25:2687–701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22:7858–61. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K, et al. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol. 2004;143:186–92. doi: 10.1038/sj.bjp.0705914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E, Marshall IC, Boyfield I, Skaper SD, Hughes JP, Owen DE, et al. Amyloid β-peptide(1–42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem. 2005;95:715–23. doi: 10.1111/j.1471-4159.2005.03396.x. [DOI] [PubMed] [Google Scholar]
- Formigli L, Sassoli C, Squecco R, Bini F, Martinesi M, Chellini F, et al. Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J Cell Sci. 2009;122:1322–33. doi: 10.1242/jcs.035402. [DOI] [PubMed] [Google Scholar]
- Fuessel S, Sickert D, Meye A, Klenk U, Schmidt U, Schmitz M, et al. Multiple tumor marker analyses (PSA, hK2, PSCA, trp-p8) in primary prostate cancers using quantitative RT-PCR. Int J Oncol. 2003;23:221–8. [PubMed] [Google Scholar]
- Ge R, Tai Y, Sun Y, Zhou K, Yang S, Cheng T, et al. Critical role of TRPC6 channels in VEGF-mediated angiogenesis. Cancer Lett. 2009;283:43–51. doi: 10.1016/j.canlet.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Gkika D, Prevarskaya N. Molecular mechanisms of TRP regulation in tumor growth and metastasis. Biochim Biophys Acta. 2009;1793:953–8. doi: 10.1016/j.bbamcr.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, et al. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–14. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, et al. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci. 2004;95:403–19. doi: 10.1254/jphs.fp0040273. [DOI] [PubMed] [Google Scholar]
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–73. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–51. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization. Trends Neurosci. 1993;16:371–6. doi: 10.1016/0166-2236(93)90095-4. [DOI] [PubMed] [Google Scholar]
- Harteneck C. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:307–14. doi: 10.1007/s00210-005-1034-x. [DOI] [PubMed] [Google Scholar]
- He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res. 2005;96:207–15. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Bindels RJ. Epithelial Ca2+ and Mg2+ channels in health and disease. J Am Soc Nephrol. 2005;16:15–26. doi: 10.1681/ASN.2004070523. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Vennekens R, Muller D, Prenen J, Droogmans G, Bindels RJ, et al. Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J Physiol. 2001;537:747–61. doi: 10.1111/j.1469-7793.2001.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–63. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Irnaten M, Blanchard-Gutton N, Harvey BJ. Rapid effects of 17beta-estradiol on epithelial TRPV6 Ca2+ channel in human T84 colonic cells. Cell Calcium. 2008;44:441–52. doi: 10.1016/j.ceca.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–60. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Kawakami S, Hara Y, Wakamori M, Itoh E, Minami T, et al. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J Pharmacol Sci. 2006;101:66–76. doi: 10.1254/jphs.fp0060128. [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–70. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- Kelly ML, Woodbury DJ. Ion channels from synaptic vesicle membrane fragments reconstituted into lipid bilayers. Biophys J. 1996;70:2593–9. doi: 10.1016/S0006-3495(96)79830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell. 2005;18:61–9. doi: 10.1016/j.molcel.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–47. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–96. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Kuhn FJ, Luckhoff A. Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2. Journal of Biological Chemistry. 2004;279:46431–7. doi: 10.1074/jbc.M407263200. [DOI] [PubMed] [Google Scholar]
- Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–26. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ. Regulation of the mouse epithelial Ca2+ channel TRPV6 by the Ca2+-sensor calmodulin. Journal of Biological Chemistry. 2004;279:28855–61. doi: 10.1074/jbc.M313637200. [DOI] [PubMed] [Google Scholar]
- Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal. 2009;2:ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees KR, Asplund K, Carolei A, Davis SM, Diener HC, Kaste M, et al. Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. GAIN International Investigators. Lancet. 2000;355:1949–54. doi: 10.1016/s0140-6736(00)02326-6. [DOI] [PubMed] [Google Scholar]
- Lehen’kyi V, Beck B, Polakowska R, Charveron M, Bordat P, Skryma R, et al. TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. Journal of Biological Chemistry. 2007a;282:22582–91. doi: 10.1074/jbc.M611398200. [DOI] [PubMed] [Google Scholar]
- Lehen’kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca2+/NFAT-dependent pathways. Oncogene. 2007b;26:7380–5. doi: 10.1038/sj.onc.1210545. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–35. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lockwich T, Singh BB, Liu X, Ambudkar IS. Stabilization of cortical actin induces internalization of transient receptor potential 3 (Trp3)-associated caveolar Ca2+ signaling complex and loss of Ca2+ influx without disruption of Trp3-inositol trisphosphate receptor association. Journal of Biological Chemistry. 2001;276:42401–8. doi: 10.1074/jbc.M106956200. [DOI] [PubMed] [Google Scholar]
- Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, et al. Functional Role of TRPV4-TRPC1 Complex in Flow-Induced Ca2+ Influx. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.109.196584. [DOI] [PubMed] [Google Scholar]
- Marasa BS, Rao JN, Zou T, Liu L, Keledjian KM, Zhang AH, et al. Induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis by inhibiting NF-κB activation through Ca2+ influx. Biochem J. 2006;397:77–87. doi: 10.1042/BJ20060124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasa BS, Xiao L, Rao JN, Zou T, Liu L, Wang J, et al. Induced TRPC1 expression increases protein phosphatase 2A sensitizing intestinal epithelial cells to apoptosis through inhibition of NF-κB activation. Am J Physiol Cell Physiol. 2008;294:C1277–C1287. doi: 10.1152/ajpcell.90635.2007. [DOI] [PubMed] [Google Scholar]
- Masuyama R, Vriens J, Voets T, Karashima Y, Owsianik G, Vennekens R, et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008;8:257–65. doi: 10.1016/j.cmet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. Journal of Biological Chemistry. 2003;278:11002–6. doi: 10.1074/jbc.M210810200. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–8. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Mery L, Strauss B, Dufour JF, Krause KH, Hoth M. The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci. 2002;115:3497–508. doi: 10.1242/jcs.115.17.3497. [DOI] [PubMed] [Google Scholar]
- Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol. 2006;209:31–41. doi: 10.1007/s00232-005-0839-3. [DOI] [PubMed] [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–72. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Minke B, Parnas M. Insights on TRP channels from in vivo studies in Drosophila. Annu Rev Physiol. 2006;68:649–84. doi: 10.1146/annurev.physiol.68.040204.100939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–7. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. 2001:1–17. doi: 10.1126/stke.2001.90.re1. http://stke.sciencemag.org/cgi/content/full/OC_sigtrans2001/90/rel. [DOI] [PubMed]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002;9:229–31. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–23. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Morris GF, Bullock R, Marshall SB, Marmarou A, Maas A, Marshall LF. Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The Selfotel Investigators. J Neurosurg. 1999;91:737–43. doi: 10.3171/jns.1999.91.5.0737. [DOI] [PubMed] [Google Scholar]
- Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T, et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. Journal of Biological Chemistry. 2007;282:32158–67. doi: 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, et al. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–5. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, et al. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics. 1998;54:124–31. doi: 10.1006/geno.1998.5551. [DOI] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–9. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- Nilius B, Vennekens R, Prenen J, Hoenderop JG, Droogmans G, Bindels RJ. The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. Journal of Biological Chemistry. 2001;276:1020–5. doi: 10.1074/jbc.M006184200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch. 2005;451:1–10. doi: 10.1007/s00424-005-1462-y. [DOI] [PubMed] [Google Scholar]
- Nishida M, Hara Y, Yoshida T, Inoue R, Mori Y. TRP channels: molecular diversity and physiological function. Microcirculation. 2006;13:535–50. doi: 10.1080/10739680600885111. [DOI] [PubMed] [Google Scholar]
- Obukhov AG, Nowycky MC. TRPC5 activation kinetics are modulated by the scaffolding protein ezrin/radixin/moesin-binding phosphoprotein-50 (EBP50) J Cell Physiol. 2004;201:227–35. doi: 10.1002/jcp.20057. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Pani B, Cornatzer E, Cornatzer W, Shin DM, Pittelkow MR, Hovnanian A, et al. Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: a potential role for TRPC1 in Darier’s disease. Mol Biol Cell. 2006;17:4446–58. doi: 10.1091/mbc.E06-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani B, Ong HL, Brazer SC, Liu X, Rauser K, Singh BB, et al. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci U S A. 2009;106:20087–92. doi: 10.1073/pnas.0905002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas M, Katz B, Lev S, Tzarfaty V, Dadon D, Gordon-Shaag A, et al. Membrane lipid modulations remove divalent open channel block from TRP-like and NMDA channels. J Neurosci. 2009;29:2371–83. doi: 10.1523/JNEUROSCI.4280-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–52. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–15. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, et al. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. Journal of Biological Chemistry. 1999;274:22739–46. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- Penner R, Fleig A. The Mg2+ and Mg2+-nucleotide-regulated channel-kinase TRPM7. Handb Exp Pharmacol. 2007:313–28. doi: 10.1007/978-3-540-34891-7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A, Suss-Toby E, Rom-Glas A, Arnon A, Payne R, Minke B. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 1994;12:1257–67. doi: 10.1016/0896-6273(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–9. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. Journal of Biological Chemistry. 2005;280:6138–48. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin- binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–42. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- Pieper AA, Verma A, Zhang J, Snyder SH. Poly (ADP-ribose) polymerase, nitric oxide and cell death. Trends Pharmacol Sci. 1999;20:171–81. doi: 10.1016/s0165-6147(99)01292-4. [DOI] [PubMed] [Google Scholar]
- Plant TD, Strotmann R. TRPV4. Handb Exp Pharmacol. 2007:189–205. doi: 10.1007/978-3-540-34891-7_11. [DOI] [PubMed] [Google Scholar]
- Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, et al. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. Journal of Biological Chemistry. 2006;281:13588–95. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R, DeRiemer SA, Sakmann B, Stadler H, Yakir N. Ion channels in synaptic vesicles from Torpedo electric organ. Proc Natl Acad Sci U S A. 1988;85:5310–4. doi: 10.1073/pnas.85.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R, Fernandez JM. Pre- and postfusion regulation of transmitter release. Neuron. 1997;18:17–27. doi: 10.1016/s0896-6273(01)80043-x. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–47. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Rohacs T. Phosphoinositide regulation of non-canonical transient receptor potential channels. Cell Calcium. 2009;45:554–65. doi: 10.1016/j.ceca.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–7. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol. 2002;4:329–36. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- Rychkov G, Barritt GJ. TRPC1 Ca2+-permeable channels in animal cells. Handb Exp Pharmacol. 2007:23–52. doi: 10.1007/978-3-540-34891-7_2. [DOI] [PubMed] [Google Scholar]
- Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–30. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528–37. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- Satoh S, Tanaka H, Ueda Y, Oyama J, Sugano M, Sumimoto H, et al. Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol Cell Biochem. 2007;294:205–15. doi: 10.1007/s11010-006-9261-0. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- Schoeber JP, Topala CN, Wang X, Diepens RJ, Lambers TT, Hoenderop JG, et al. RGS2 inhibits the epithelial Ca2+ channel TRPV6. Journal of Biological Chemistry. 2006;281:29669–74. doi: 10.1074/jbc.M606233200. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Watt JA, Singh BB. TRPC1 inhibits apoptotic cell degeneration induced by dopaminergic neurotoxin MPTP/MPP(+) Cell Calcium. 2009;46:209–18. doi: 10.1016/j.ceca.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan D, Marchase RB, Chatham JC. Overexpression of TRPC3 increases apoptosis but not necrosis in response to ischemia-reperfusion in adult mouse cardiomyocytes. Am J Physiol Cell Physiol. 2008;294:C833–C841. doi: 10.1152/ajpcell.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J, Beck A, Fleig A, Penner R. Regulation of TRPM2 by extra- and intracellular calcium. J Gen Physiol. 2007;130:427–40. doi: 10.1085/jgp.200709836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci. 2009;12:1300–7. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- Takuwa N, Takuwa Y. Signal transduction of cell-cycle regulation: its temporo-spacial architecture. Jpn J Physiol. 1996;46:431–49. doi: 10.2170/jjphysiol.46.431. [DOI] [PubMed] [Google Scholar]
- Takuwa N, Zhou W, Takuwa Y. Calcium, calmodulin and cell cycle progression. Cell Signal. 1995;7:93–104. doi: 10.1016/0898-6568(94)00074-l. [DOI] [PubMed] [Google Scholar]
- Thebault S, Flourakis M, Vanoverberghe K, Vandermoere F, Roudbaraki M, Lehen’kyi V, et al. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006;66:2038–47. doi: 10.1158/0008-5472.CAN-05-0376. [DOI] [PubMed] [Google Scholar]
- Tong Q, Zhang W, Conrad K, Mostoller K, Cheung JY, Peterson BZ, et al. Regulation of the transient receptor potential channel TRPM2 by the Ca2+ sensor calmodulin. Journal of Biological Chemistry. 2006;281:9076–85. doi: 10.1074/jbc.M510422200. [DOI] [PubMed] [Google Scholar]
- Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61:3760–9. [PubMed] [Google Scholar]
- van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, et al. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- Voets T, Janssens A, Prenen J, Droogmans G, Nilius B. Mg2+-dependent Gating and Strong Inward Rectification of the Cation Channel TRPV6. J Gen Physiol. 2003;121:245–60. doi: 10.1085/jgp.20028752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Prenen J, Fleig A, Vennekens R, Watanabe H, Hoenderop JG, et al. CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. Journal of Biological Chemistry. 2001;276:47767–70. doi: 10.1074/jbc.C100607200. [DOI] [PubMed] [Google Scholar]
- Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, et al. Molecular determinants of permeation through the cation channel TRPV4. Journal of Biological Chemistry. 2002;277:33704–10. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- Walder RY, Yang B, Stokes JB, Kirby PA, Cao X, Shi P, et al. Mice defective in TRPM6 show embryonic mortality and neural tube defects. Hum Mol Genet. 2009;18:4367–75. doi: 10.1093/hmg/ddp392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xu H, Oberwinkler J, Gu Y, Hardie RC, Montell C. Light activation, adaptation, and cell survival functions of the Na+/Ca2+ exchanger CalX. Neuron. 2005;45:367–78. doi: 10.1016/j.neuron.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. Journal of Biological Chemistry. 2002a;277:13569–77. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–8. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. Journal of Biological Chemistry. 2002b;277:47044–51. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. Journal of Biological Chemistry. 2002;277:23150–6. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- Weick JP, Johnson MA, Zahng S-C. Developmental regulation of human embryonic stem cell-derived neurons by calcium entry via transient receptor potential channels. Stem Cells. 2009:2906–16. doi: 10.1002/stem.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci, USA. 1995;92:9652–6. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissenbach U, Bodding M, Freichel M, Flockerzi V. Trp12, a novel Trp related protein from kidney. FEBS Lett. 2000;485:127–34. doi: 10.1016/s0014-5793(00)02212-2. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Niemeyer BA. TRPV6. Handb Exp Pharmacol. 2007:221–34. doi: 10.1007/978-3-540-34891-7_13. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, et al. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. Journal of Biological Chemistry. 2001;276:19461–8. doi: 10.1074/jbc.M009895200. [DOI] [PubMed] [Google Scholar]
- Wu X, Zagranichnaya TK, Gurda GT, Eves EM, Villereal ML. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. Journal of Biological Chemistry. 2004;279:43392–402. doi: 10.1074/jbc.M408959200. [DOI] [PubMed] [Google Scholar]
- Yoon J, Cohen Ben-Ami H, Hong YS, Park S, Strong LLR, Bowman J, et al. Novel mechanism of massive photoreceptor degeneration caused by mutations in the trp gene of Drosophila. J Neurosci. 2000;20:649–59. doi: 10.1523/JNEUROSCI.20-02-00649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, et al. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2004;101:13861–6. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, et al. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003;284:C316–C330. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–89. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–73. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- Zhang S, Remillard CV, Fantozzi I, Yuan JX. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2004;287:C1192–C1201. doi: 10.1152/ajpcell.00158.2004. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, et al. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. Journal of Biological Chemistry. 2003;278:16222–9. doi: 10.1074/jbc.M300298200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Hirschler-Laszkiewicz I, Tong Q, Conrad K, Sun SC, Penn L, et al. TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am J Physiol Cell Physiol. 2006;290:C1146–C1159. doi: 10.1152/ajpcell.00205.2005. [DOI] [PubMed] [Google Scholar]
- Zhao H, Grossman HB, Spitz MR, Lerner SP, Zhang K, Wu X. Plasma levels of insulin-like growth factor-1 and binding protein-3, and their association with bladder cancer risk. J Urol. 2003;169:714–7. doi: 10.1097/01.ju.0000036380.10325.2a. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–8. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, et al. Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Invest. 2002;82:1755–64. doi: 10.1097/01.lab.0000043910.41414.e7. [DOI] [PubMed] [Google Scholar]