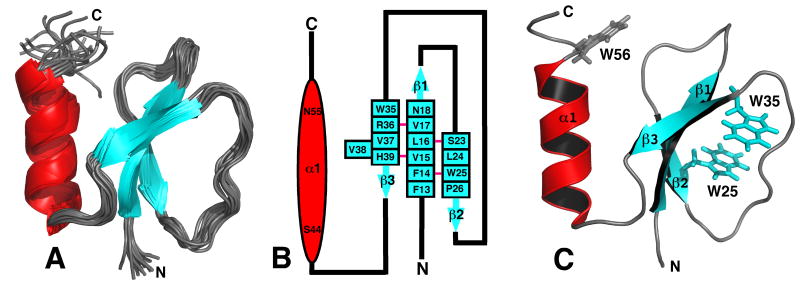
Figure 2.
A) Superposition of the ensemble of 20 lowest energy structures calculated for Rv2377c. Because both the N- and C-terminal regions are disordered, G1* - A12 and W56 - D71, respectively, most of these regions have been removed from the Figure for clarity. B) Secondary structure diagram of Rv2377c. The α-helix is drawn as a red ovals and the β-strands as solid blue arrows with the residue number at the beginning and the end of each structural element shown. The β-sheet contains a bulge at residue V38. A solid pink line between the β-strand residues indicates dual hydrogen bonds between two residues in an antiparallel β-sheet. C) Ribbon representation of the structure in the ensemble closest to the average structure for Rv2377c highlighting the position of the three exclusively conserved tryptophan residues in the MbtH-like family of proteins. While the side chains of W25 and W35 superimpose well in the ensemble, W56 assumes many positions. The β-strands are colored blue and the α-helix is colored red.