Figure 3.
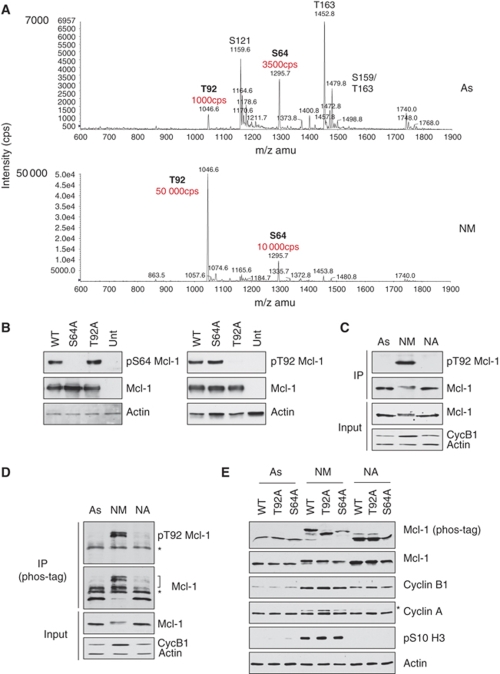
Mcl-1 is phosphorylated at two sites in mitosis. (A) Flag-Mcl-1 was immunoprecipitated from HeLa cells growing in asynchronous culture (As) or treated with nocodazole for 3 h, followed by collection of the rounded-up, mitotic cells (NM). Tryptic peptides were analysed by mass spectrometry. The relative abundance of the phosphopeptides containing the Thr92 and Ser64 sites are highlighted. (B) Specificity of antibodies generated against Mcl-1 phosphorylation sites. HeLa cells were transiently transfected with either wild-type Mcl-1 (WT) or the phosphorylation site mutants S64A or T92A. After treatment with 250 ng/ml nocodazole for 2 h, samples were analysed by SDS–PAGE and immunoblotting with antibodies raised either against the phosphorylated Ser64 (left) or Thr92 (right) sites. (C, D) Mcl-1 is phosphorylated at Thr92 in mitosis. Endogenous Mcl-1 was immunoprecipitated from asynchronous (As) HeLa cells or after treatment with 100 ng/ml nocodazole for 2 h before separation of mitotic (NM) and adherent cells (NA). Cell lysates (Input) were analysed by conventional SDS–PAGE. Immunoprecipitates were analysed by conventional SDS–PAGE (C) or Phos-tag SDS–PAGE (D) and immunoblotted for Mcl-1 phosphorylated at Thr92 or total Mcl-1. Phosphorylated Mcl-1 is indicated by a bracket. *Indicates the heavy chain of the precipitating antibody. (E) Mcl-1 is highly phosphorylated at Thr92 and Ser64 during mitotic arrest. U2OS cells stably transfected with either wild-type Mcl-1 (WT), T92A or S64A mutants were untreated (As) or treated for 2 h with nocodazole before separation of mitotic (NM) and adherent cells (NA). Cell lysates were analysed by conventional SDS–PAGE or Phos-tag SDS–PAGE as indicated and immunoblotted with the specified antibodies. *Indicates a band corresponding to cyclin B1 caused by reprobing of the same blot used to identify cyclin B1.