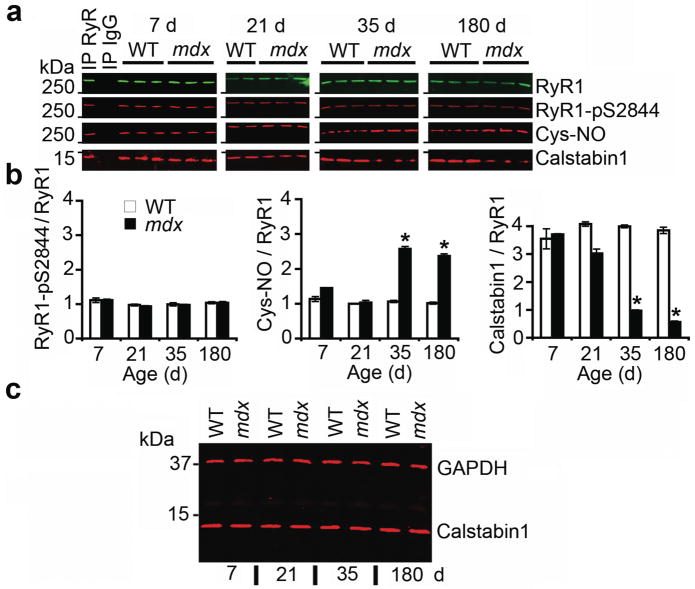
Figure 1. RyR1 is S-nitrosylated and depleted of calstabin1 in mdx mice.
(a) RyR1 was immunoprecipitated from EDL muscle of mdx mice and WT littermates at 7, 21, 35, and 180 days after birth and immunoblotted for RyR1, RyR1 PKA phosphorylated at Ser2844 (RyR1-pS2844), S-nitrosylation of cysteine residues on RyR1 (Cys-NO), and calstabin1 bound to RyR1. Positive and negative control (IgG) immunoprecipitation are shown for 7 day WT hearts. Blots are representative of three independent experiments. (b) Quantification of RyR1 PKA phosphorylation, RyR1 S-nitrosylation, and bound calstabin1 relative to total RyR1. Data presented as mean ± S.E.M. *, P < 0.05, t-test. (c) Immunoblot for total calstabin1 in whole EDL muscle lysate (25 μg) from WT and mdx mice at indicated ages. GAPDH was used as a loading control.