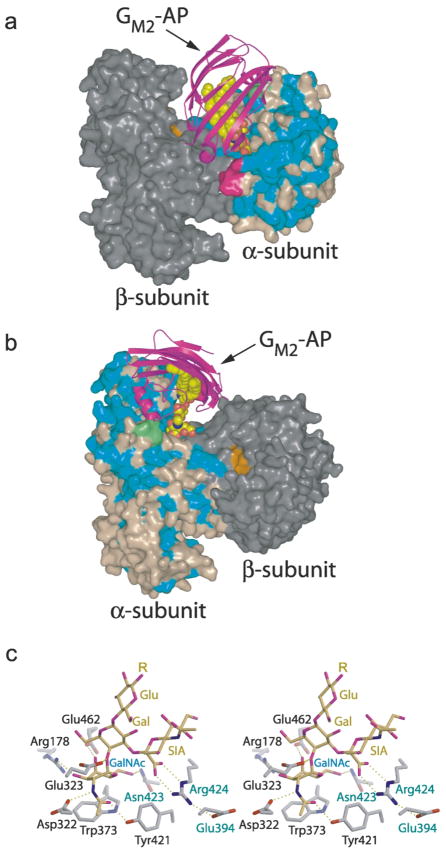
Figure 6.
Predicted model of human Hex A–GM2–activator quaternary complex. (a and b) Two views of the predicted quaternary complex. Residues of the α-subunit identical to those of the β-subunit are colored blue, non-identical residues are colored light brown. Most of the conserved amino acids in the α and β-subunits are located in (β/α)8-barrel of domain II. The β-subunit is colored gray, with residues of the active site distinguished in orange. The GM2–activator protein complex (GM2–AP) docks into a large groove between the two subunits so that the terminal non-reducing GalNAc sugar on GM2 can be presented to the α-subunit active site and removed. Two surface loops (magenta and green), present only on the α-subunit, interact with the docked activator protein and appear to be involved in creating a docking site unique to the α-subunit. The magenta colored loop is proteolytically removed from the β-subunit during post-translational processing and may represent a modification that regulates the metabolic function of this subunit. (c) Model of the GM2 oligosaccharide (yellow) bound to the α-subunit active site (gray). The distorted boat conformation of the terminal GalNAc to be removed (Gal, labeled in blue) and the pseudoaxial orientation of the scissile bond and leaving group are based on crystallographic observations of the Michaelis complex of chitobiose bound to SmCHB.20 By incorporating these conformational restraints into the model, only one reasonable position could be found for the sialic acid residue (labeled SIA) within the active site pocket. Once positioned, the negatively charged carboxylate of the sialic acid, which can only be accommodated by the α-subunit, was found to come within hydrogen bonding distance of Arg424, a positively charged residue that is unique to the α-subunit (the β-subunit contains a Leu at this position). αGlu394 and αAsn423 (which are both Asp residues in the β-subunit) are believed to help hold Arg424 into position. Arg424, in turn, stabilizes the negatively charged caboxylate of the sialic acid of the substrate via electrostatic and hydrogen-bonding interactions. The general acid–base residue, Glu323 (Glu355 in the β-subunit), can be seen interacting with the glycosidic oxygen atom of the scissile bond.