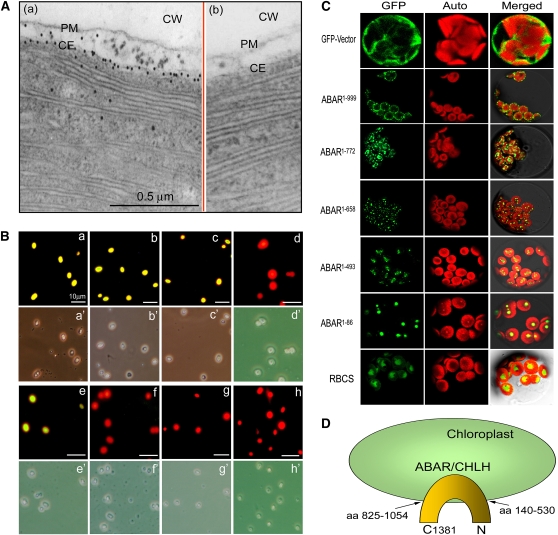
Figure 2.
ABAR Spans the Chloroplast Envelope and Is Exposed to Cytosol with Its N and C Termini.
(A) Immunogold electronic microscopy shows that ABAR (visualized by gold particles) predominantly localizes in chloroplast envelope membranes (a). Panel (b) shows a control where the purified IgG of rabbit preimmune serum was used instead of the anti-ABAR serum in the immunolabeling, and no signal was detected. CW, cell wall; PM, plasma membrane; CE, chloroplast envelope.
(B) Immunodetection in the isolated, intact chloroplasts shows that ABAR spans the chloroplast envelope and is exposed to cytosol with its N and C terminus. The anti-ABAR (a), anti-ABAR-C terminus (b), and anti-ABAR-N terminus (c) sera recognize immunosignal (marked by yellow-green fluorescence), while the anti-ABAR middle fragment serum (d) does not. Note that the anti-Toc34 (outer envelope marker) detects immunosignal (e), but the anti-Tic40 (inner envelope marker; [f]) and anti-Hcf101 (stroma marker; [g]) sera and the purified IgG of the rabbit preimmune serum (h) do not detect signal. The corresponding bright field is displayed below each fluorescence image and indicated by the same letter marked by an apostrophe.
(C) Subcellular localization of the truncated ABARs. RBCS was used as a stroma marker. The superscript numbers indicate the numbers of amino acid residues in the order from the N terminus to the truncation site. GFP, Auto, and Merged indicate fluorescence of the truncated ABAR-GFP or RBCS-GFP fusion protein, chlorophyll autofluorescence, and merged image of GFP and Auto in the bright field, respectively.
(D) A model showing that ABAR is predominantly a trans-chloroplast membrane protein. aa 140-530 and aa 825-1052 indicate predicated transmembrane domains at N and C termini, respectively (see Supplemental Figure 1 online). C1381, the amino acid residue 1381 in the ABAR C end.