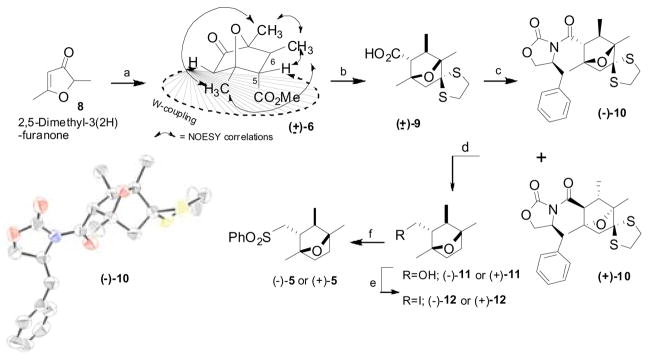
Scheme 2. aSynthesis of chiral 7-oxabicyclo[2.2.1]-heptane ring system.
aReagents and conditions: a) LDA, Et2O:cyclohexane, −78 °C, then methyl crotonate, 69%; b) i. Ethanedithiol, p-TsOH, benzene, reflux; ii. LiOH, aq. MeOH, 92%, two steps; c) TEA, trimethylacetyl chloride, LiCl, (S)-(−)-4-benzyl-2-oxazolidinone, CH2Cl2, rt, separation of diastereomers by silica gel chromatography: (−)-10, 40%, (+)-10, 42%; d) i. Raney Ni, EtOH, reflux, 98%; ii. LiBH4, THF, 0 °C, (−)-11, 95%, (+)-11 96%; e) Ph3P, I2, imidazole, CH2Cl2, rt, (−)-12, 94%, (+)-12, 92%; f) PhSO2Na, DMF, 60 °C, (−)-5, 90%, (+)-5, 91%. ORTEP representation of the N-acyloxazolidinone (−)-10 (H atoms are omitted for clarity)