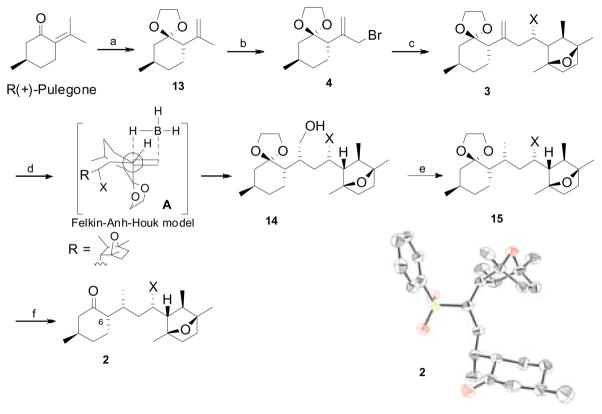
Scheme 3. aFragment coupling and synthesis of keto-sulfone 2 (X = SO2Ph).
aReagents and conditions; a) (CH2OH)2, CSA, benzene, reflux, 24 h, 73% (dr 92:8); b) NBS, Yb(OTf)3, TMSCl, CH2Cl2, 0 °C, 15 min, 48%; c) (−)-5, n-BuLi, HMPA, THF, −40 °C, 40 min, then 4, 2 h, 98% (dr 95:5); d) i. BH3.S(CH3)2, THF, 10 h, ii. H2O2, NaOH, 0 °C, 87%; e) i. PhSSPh, (n-Bu3)P, toluene, rt, 94%; ii. Raney Ni, EtOH, reflux, 90%; f). PdCl2(CH3CN)2, acetone, rt, 30 min, 92%. In ORTEP representation of one of the two crystallographically independent molecules of 2 (H atoms and cocrystallized ether molecules are omitted for clarity).