Abstract
One small previous study found that praziquantel reduced hookworm infection. In this study, 607 subjects were enrolled in a longitudinal study. At enrollment and every 3 months for 18 months, three stool samples were collected, and the intensity of infection with Schistosoma japonicum and soil-transmitted helminths (STHs) was quantified. All subjects were treated with 60 mg/kg praziquantel at baseline. Three months post-treatment, the percent of subjects who were hookworm-infected decreased to 46.5% from 61% at baseline. The putative cure rate was 23.7%. The 95% confidence interval around the change in hookworm egg counts from baseline to 6 and 12 months post-treatment was negative and did not include zero. The percent reduction in hookworm egg counts from baseline to 3 months post-treatment was 40.8%. Praziquantel treatment did not decrease the infection intensity of STHs. Control programs using praziquantel may have the added benefit of reducing hookworm infection and anemia in areas of coendemnicity.
Introduction
Hookworm infection is a leading cause of iron-deficiency anemia that afflicts an estimated 576 million people throughout developing nations of the tropics, with highest prevalence in East Asia/Pacific Islands and sub-Saharan Africa.1 The global burden of hookworm is an estimated 1.5–22.1 million disability-adjusted life years annually.2 Albendazole is currently accepted as the most effective treatment of hookworm infection, with a single dose providing egg reduction rates of 64.2–100% and cure rates of about 78.4%.3
Praziquantel is known to be effective in treating schistosomiasis in humans as well as a wide range of trematodes and human and veterinary cestodes.4 After oral administration, praziquantel is quantitatively and rapidly absorbed, metabolized, and excreted as a variety of metabolites, predominantly through the kidneys.5 Although the mechanism of action of praziquantel is not entirely clear, it is thought that the drug acts on the muscle fibers of schistosomes, inducing paralysis.6 Other work suggests that praziquantel leads to unmasking of antigens on the worm surface, making them more accessible to host immune cells.7 To our knowledge, no studies have assessed the in vitro activity of praziquantel against soil-transmitted helminths (STHs), specifically Ascaris lumbricoides, Trichuris trichuira, and hookworm.
One population-based study, conducted in an area of the Ivory Coast highly endemic for Schistosoma mansoni and hookworm, examined the in vivo effect of praziquantel against hookworm infection.4 In that study conducted among 96 school children, a significant reduction in the prevalence and intensity of hookworm infection was found 4 weeks after treatment with praziquantel. To our knowledge, this is the only population study to have examined the efficacy of praziquantel against hookworm.
Praziquantel has become increasingly available, with an average cost of US $0.40 per dose, and a worldwide initiative is underway to provide annual mass treatment in high-risk communities.8
In areas of the developing world endemic for helminths, polyparasitic infections are normal, with most children harboring two or more helminths rather than a single infection.9–14 STHs and schistosomes are the most common helminth infections. Of these infections, hookworm and schistosome infections have been most clearly implicated as causes of anemia, particularly among children.15–19
This study was conducted among 607 S. japonicum infected 7- to 30-year-old males and non-pregnant females in Leyte, the Philippines. The objectives of this study were to investigate the efficacy of praziquantel against hookworm in a different ecological setting and to extend previous findings by examining praziquantel's efficacy using longitudinal data, a larger sample, and a larger dose of praziquantel.
Methods
Study population.
This longitudinal study was conducted in three rice-farming villages in Leyte, the Philippines (Macanip, Buri, and Pitogo), where both S. japonicum and STHs are endemic. Three to six months before the start of enrollment, a census was completed in each village. Study staff went door to door accompanied by a resident of the village, mapping each household with global positioning satellite devices. The census enumerated number of individuals in the household, their gender, and dates of birth. At that time, all individuals in the household ages 8–30 were asked to provide informed consent for the stool-screening procedures for a study investigating immune correlates of reinfection, the original objective of this work. Subjects were eligible for participation if they were infected with S. japonicum, lived primarily in a study village, were 7- to 30-years-old, were not pregnant or lactating, and provided both child assent and parental consent or adult consent. Subjects with severe hepatomegaly or grade II or higher hepatic fibrosis on ultrasound examination, as well as subjects with severe anemia (hemoglobin < 7.0 g/dL) or severe wasting (weight for height Z score < −3.0), were excluded from participation and referred for medical treatment. This group was treated for S. japonicum and STHs, and iron was provided for anemic subjects. The group of subjects with grade II or higher hepatic fibrosis was also placed on a 2-year praziquantel treatment protocol, with stool screening and treatment every 3 months. Finally, hepatic fibrosis was reassessed by ultrasound at 12 months post-treatment for all enrolled subjects. Subjects who had newly developed grade II or higher fibrosis at this time were withdrawn from the study and placed on the aforementioned treatment protocol.
All eligible subjects were treated with a split dose of praziquantel (60 mg/kg). Enrolled subjects were not treated for STHs at baseline. This was because of the fact that treatment of STHs could modify immune responses to S. japonicum and limit interpretability of baseline responses to S. japonicum antigens and their relationship to reinfection risk. Subsequently, participants were followed at 3, 6, 9, 12, 15, and 18 months post-treatment. At each time point, stool samples were collected, and a physical examination was performed. All participants were transported to the study clinic by study staff for enrollment and follow-up visits. All subjects infected with STHs or S. japonicum were treated at the end of the study with albendazole and/or praziquantel, respectively. Subjects could not be treated for S. japonicum earlier, given that the main objective of this work was to identify immune correlates of reinfection, and sufficient time was needed to allow variability in this outcome. Brown University and The Philippines Research Institute of Tropical Medicine institutional review boards approved this study.
Stool examination.
Two weeks before each follow-up visit, an attempt was made to collect three consecutive stool samples from each participant at their home. Parasite egg counts were determined by examination of consecutive stool specimens obtained from each study participant on separate days. Each of the stool specimens was examined in duplicate for S. japonicum, A. lumbricoides, T. trichiura, and hookworm eggs within 24 hours of collection by the Kato–Katz method. Egg counts were enumerated within 1 hour of preparation for hookworm, and this protocol was followed throughout the study. The mean eggs per gram (epg) of stool was used as the quantitative measure of infection status for each worm based on the average egg count for each duplicate specimen. Then, the mean egg count was taken across the stool samples provided, including samples with 0 epg enumerated. World Health Organization criteria were used to classify each infection as uninfected or low, moderate, or high intensity as follows: hookworm: low = 1–1,999 epg, moderate = 2,000–3,999 epg, high ≥ 4,000 epg; S. japonicum: low = 1–99 epg, moderate = 100–399 epg, high ≥ 400 epg; A. lumbricoides: low = 1–4,999 epg, moderate = 5,000–49,999 epg, high ≥ 50,000 epg; T. trichiura: low = 1–999 epg, moderate = 1,000–9,999 epg, high ≥ 10,000 epg.20 Ten hookworm larvae, obtained by culturing stool samples21 from 203 study participants, were speciated by polymerase chain reaction (PCR); only Necator americanus was detected, and Ancylostoma duodenale species were not detected.
Statistical analyses.
For all covariates, initial assessment of normality was performed using standard normality diagnostics including skewness, kurtosis, and the Shapiro–Wilks test. Cure rate was calculated as percent infected at baseline minus percent infected at follow-up divided by percent infected at baseline. S. japonicum and STH egg counts were right skewed and not normally distributed. Egg counts were, therefore, loge transformed [ln (n + 1)] to produce a geometric mean egg count.
Proc mixed in SAS version 9.1 was used to calculate mean geometric egg count at each time point for each STH while adjusting for lack of independence of responses because of both (1) repeated measures within individuals and (2) individuals living in the same household. The least-square means procedure was used to derive the mean geometric egg count with 95% confidence intervals at each time point for each STH while adjusting for other covariates and clustering by household. We also assessed interaction effects by gender and age strata (both age < 13 years and age < 18 years) by creating a time × gender (or time × age strata) covariate.
Given that the proc mixed approach uses available data from each subject at each time point but assumes any missing data is missing at random, we did one further analysis to guard against bias in loss to follow-up, which could violate the assumption of missing data at random. This could, in turn, affect the sample mean egg counts over time. Within-person change scores were created for each STH per time point of measurement relative to baseline using the geometric mean epg. This change from baseline, therefore, only includes individuals who provided stool at baseline and the specific follow-up time point. We then evaluated whether 95% confidence intervals around the change at each time point did or did not include zero, with inclusion of zero implying a non-significant change from baseline. This analysis was also adjusted for clustering of responses by individuals from the same household.
Results
Overall, 74.3% (1,262/1,699) of individuals between the ages of 7 and 30 years residing in study villages were screened for the presence of S. japonicum, hookworm, A. lumbicoides, and T. trichiura. Of these, 60% were infected with S. japonicum and eligible for further screening. In total, 607 individuals ultimately met all eligibility criteria, with most exclusions caused by lack of S. japonicum infection. These individuals were enrolled and treated with praziquantel. At baseline, 39.0% had no hookworm infection, 58.0% had low-intensity infection, 2.31% had moderate-intensity infection, and 0.66% had high-intensity infection. Other baseline characteristics of the cohort are presented in Table 1.
Table 1.
Baseline characteristics of the study cohort
| Characteristic | Value |
|---|---|
| Age (years; mean [standard deviation]) | 15.0 (6.18) |
| Sex (number of males/total subjects; %) | 383/607 (63.1) |
| Hookworm prevalence (%) | 61.0 |
| Hookworm infection intensity* | |
| No infection (%) | 39.0 |
| Low intensity (%) | 58.0 |
| Moderate intensity (%) | 2.30 |
| High intensity (%) | 0.70 |
| Hookworm median eggs per gram of stool | 60.0 |
| S. japonicum prevalence (%) | 100 |
| S. japonicum infection intensity* | |
| No infection (%) | 0 |
| Low intensity (%) | 71.7 |
| Moderate intensity (%) | 22.7 |
| High intensity (%) | 5.6 |
| S. japonicum median eggs per gram of stool | 40.0 |
| A. lumbricoides prevalence (%) | 76.0 |
| A. lumbricoides infection intensity* | |
| No infection (%) | 24.0 |
| Low intensity (%) | 25.7 |
| Moderate intensity (%) | 38.6 |
| High intensity (%) | 11.7 |
| A. lumbricoides median eggs per gram of stool | 5,133.3 |
| T. trichuria prevalence (%) | 92.2 |
| T. trichuria infection intensity* | |
| No infection (%) | 7.8 |
| Low intensity (%) | 50.2 |
| Moderate intensity (%) | 38.1 |
| High intensity (%) | 3.9 |
| T. trichuria median eggs per gram of stool | 800 |
| Hemoglobin g/dL (mean [standard deviation]) | 12.5 (1.8) |
Hookworm: low = 1–1,999 eggs per gram of stool (epg), moderate = 2,000–3,999 epg, high ≥ 4,000 epg. S. japonicum: low = 1–99 epg, moderate = 100–399 epg, high ≥ 400 epg. A. lumbricoides: low = 1–4,999 epg, moderate = 5,000–49,999 epg, high ≥ 50,000 epg. T. trichuria: low = 1–999 epg, moderate = 1,000–9,999 epg, high ≥ 10,000 epg.
Infection intensities based World Health Organization criteria.20
Between 79.4% and 86.8% of subjects were present at each time point. Given that the sensitivity of the Kato–Katz method is affected by the number of stools examined22–24 and the concern that the sample prevalence of infection would appear to decline if subjects provided fewer stools over time, we quantified the number of stools provided at each time point. This was calculated for those who provided stool, because the prevalence of infection after treatment is only evaluated among those who provided at least one stool. A larger percentage of subjects provided three stools at later time points compared with baseline: 3 months post-treatment compared with baseline (82.2% versus 58.0%, respectively; χ2, P = 0.06) and 6 months post-treatment compared with baseline (78.1% versus 58.0%, respectively; χ2, P < 0.05).
Three months post-treatment, the percent of subjects who were hookworm-infected (any level of intensity) decreased to 46.5% from 61% at baseline (Figure 1). The cure rate was 23.7%. Comparing percentage of subjects in specific hookworm-intensity groups at baseline with both 3 and 6 months post-treatment groups, there was a significant increase in the percent of subjects in lower intensity groups at follow-up time points (χ2, P < 0.0001 for both comparisons). The median absolute hookworm egg counts were 60 epg at baseline and 0 epg at both 3 and 6 months post-treatment (Wilcoxon signed rank, P < 0.0001 for both comparisons).
Figure 1.
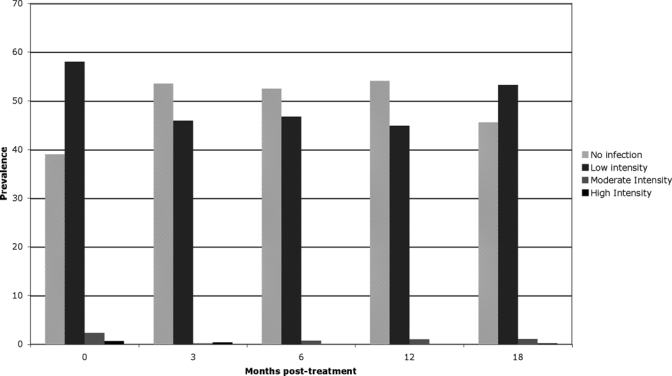
Prevalence of hookworm infection by intensity and time point.
Figure 2 presents the least-square mean geometric mean egg count for each STH at baseline (just before treatment) and subsequent time points. Geometric mean hookworm egg counts dropped significantly from baseline to each subsequent time point (least-square means difference, P < 0.0001 for all comparisons). For A. lumbicoides and T. trichiura, the only significant difference in geometric mean egg counts compared with baseline was for T. trichiura at 18 months post-treatment (least-square means difference, P < 0.001).
Figure 2.
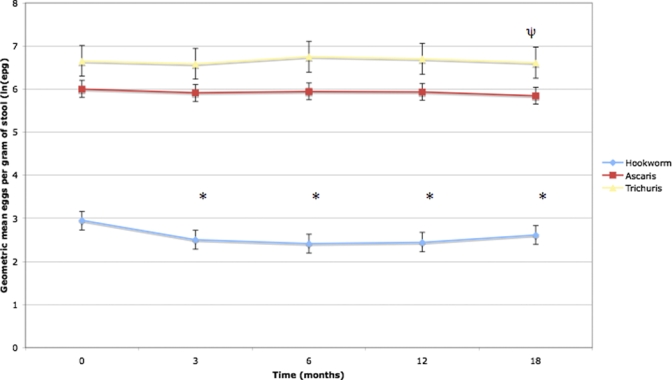
Mean geometric mean egg count at baseline and follow-up time points for hookworm, Ascaris, and Trichuris. Error bars represent 95% confidence intervals. * P < 0.0001 compared with value at month 0 (baseline). ψ P < 0.001 compared with value at month 0 (baseline). This figure appears in color at www.ajtmh.org.
Figure 3 presents the mean within-person change in geometric mean egg counts from baseline to successive time points. Each of these changes for hookworm is negative, suggesting a decrease from baseline that reaches a maximum decrease at 6 months and then begins to increase by 18 months, possibly because of newly acquired infections. Of note, the 95% confidence intervals for this change do not include zero for any of the hookworm changes in egg counts, implying that the change from baseline intensity was significantly greater than zero. The percent reduction in geometric mean egg count from baseline to 6 months, including those uninfected at baseline, was 40.8% (34.7–47.0%; 95% confidence interval). All of the 95% confidence intervals for the mean change in geometric mean egg count for A. lumbicoides and T. trichiura include zero, with the exception of T. trichiura at 18 months post-treatment. This implies that the egg counts for these two STHs were not significantly different from baseline.
Figure 3.
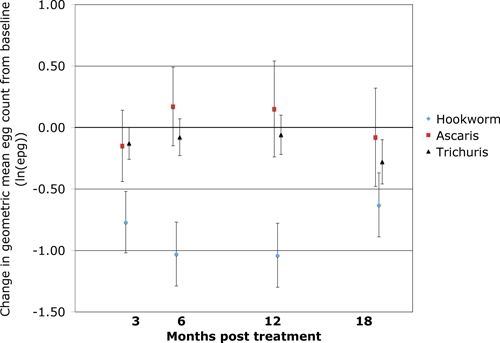
Mean within-person change in geometric mean egg count from baseline to follow-up time points for hookworm, Ascaris, and Trichuris. Error bars represent 95% confidence intervals. This figure appears in color at www.ajtmh.org.
Finally, we examined interactions between change in hookworm-infection intensity and both age (< 13 and < 18 years in separate analyses) and gender, and we did not find significant interaction effects.
Discussion
In this cohort of 607 S. japonicum-infected 7- to 30-year-olds in Leyte, the Philippines, treatment with praziquantel was followed by a reduction in hookworm prevalence and intensity of infection for up to 12 months, with prevalence and intensity at consistently lower levels than baseline for 12 months after treatment. Of note, the dose of praziquantel used in this study was the standard dose for S. japonicum (60 mg/kg), which is higher than the standard dose used for S. mansoni or S. haematobium (40 mg/kg). At 18 months post-treatment, hookworm prevalence and infection intensity began to rise again, likely because of reinfection. No effects of praziquantel treatment at baseline were seen for T. trichiura or A. lumbicoides.
Praziquantel's mechanism of action against schistosomes is not fully understood, and to our knowledge, there has been no research examining its in vitro efficacy against hookworm or other STHs. Blair and others6 found that praziquantel acts on the muscle fibers of schistosomes, inducing paralysis thought to be related to calcium-channel activity. If this is the primary mechanism through which praziquantel affects hookworms, it could act during the migratory or blood stage of infection as praziquantel is rapidly absorbed into the bloodstream. Alternatively, unabsorbed praziquantel may have some activity against adult worms in the gut lumen. If this is the mechanism, it is likely that praziquantel acts against muscle proteins shared by schistosomes and hookworms but not A. lumbicoides or T. trichuris. Given the rapid fall in egg counts by 3 months, some activity against the adult worm is likely, because the time from larval stage to patent infection with ovo-deposition is over 40 days for hookworms. In addition, a recent study from Brazil showed a synergistic effect of hookworm and S. mansoni. Specifically, a significant positive association for coinfection with hookworm and S. mansoni was shown. A change in the cytokine profile after treatment with praziquantel that would favor the host immune response to hookworm infections could potentially explain the decrease in hookworm prevalence.
Previous studies have noted improvements in hemoglobin after treatment with praziquantel, particularly in children. Given the rapid increase after treatment without provision of iron in most studies, much of that improvement is likely caused by amelioration of S. japonicum-related anemia of inflammation.16,25 It is possible, however, that some of the sustained improvement in hemoglobin found in other studies may be caused by reduction in hookworm burden. Interestingly, a previous study conducted in Leyte, The Philippines, showed that coinfection with S. japonicum and hookworm had a synergistic effect on anemia burden in children, meaning that the effect of having both infections was greater than would be expected if their effects were purely additive.14 If praziquantel does have some efficacy against hookworm, its impact on anemia may be greater than anticipated if it has some impact on two helminths with synergistic effects on anemia. Furthermore, although most studies have shown that the risk of anemia is much greater among children with moderate- to high-intensity hookworm infections, the presence of multiple low-intensity infections with helminths also increases the risk for anemia.26 Given the very high prevalence of low-intensity helminth infections globally, praziquantel may further limit anemia-related morbidity by reducing the number of children coinfected with multiple helminths at low intensity.
The World Health Organization (WHO) has endorsed regular administration of anti-helminthic drugs to control morbidity related to STHs. In high-risk communities (prevalence of any STH among school-aged children > 50%), current treatment guidelines call for twice yearly treatment of all school-aged children as well as treatment of pre-school–aged children, pregnant women in second or third trimester, and adults in high-risk groups.8 In areas where hookworm and schistosomiasis are coendemic, joint therapy of albendzole and praziquantel is recommended, with schedules related to community prevalence.8 In many lesser developed countries (LDCs) however, even provision of one drug annually is difficult to achieve. Until treatment targets are met for both STHs and schistosomiasis, communities with coendemnicity that are benefiting from current schistosomiasis-control programs27 but still have suboptimal coverage for STHs may accrue additional benefits by limiting the intensity of hookworm infection with praziquantel.
The most important limitation of this study is the lack of an untreated control group. In a previous four-arm randomized controlled trial of albendazole and praziquantel, there was a fall in the prevalence of hookworm infection 45 days post-treatment in the placebo group, which did not reach statistical significance.28 No data were provided with respect to change in intensity of hookworm infection in that time. Thus, it is possible that the observed decreases in hookworm infection were caused by changes in the ecology of transmission, improvements of sanitation and hygiene, or unmeasured treatment with albendazole. These are unlikely. During the time course of the study, there were no major changes in climate or natural disasters such as floods or typhoons that might disrupt transmission. In addition, no large-scale improvements in sanitation were made during the study, and such improvements would be expected to impact the transmission of ascaris and Trichuris, both of which are also related to poor sanitation and hygiene. Participants' health was monitored for 18 months after praziquantel treatment as part of the clinical and ethical obligations of the study, with active assessments every 3 months. We had no systematic evidence or anecdotal reports of participants seeking or receiving care for the STHs.
An additional limitation is the use of egg counts as a proxy measure for worm burden and expected morbidity. It is possible that praziquantel impacted adult worm fecundity rather than survival. If this is the case, praziquantel's expected impact on morbidity caused by hookworm is likely to be smaller than expected. Small numbers of subjects with moderate- and high-intensity infection limit the inferences one can make on praziquantel's efficacy in the context of higher-intensity hookworm infections. Finally, all stool exams for worm eggs have some degree of measurement error, although for hookworm, accuracy improves with number of stool specimens.22,29,30 We think this is less of a problem, because a large proportion of the sampled population provided three stool specimens at most follow-up visits, the number of stools provided at each time point was greater than at baseline, and we used the same procedure for collecting, preparing, and reading stool samples throughout the course of the study.
Ongoing and upcoming operational research studies such as Schistosomiasis Control Initiative (SCI), Schistosomiasis Consortium for Operational Research and Evaluation (SCORE), and the multidisciplinary alliance, CONTRAST, which aim to inform the best approaches to praziquantel delivery, including frequency of treatment, target populations, and treatment locations (e.g., school- versus community-based), may continue to increase the number of doses of praziquantel provided in sub-Saharan Africa.27 These and other future studies should examine this issue with a randomized controlled design across other settings, including areas with Ancylostoma duodenale. Quantification of praziquantel's ability to limit hookworm-related anemia conducted in areas without schistosomiasis would help elucidate its potential contribution to control of this important morbidity. If other studies support the ability of praziquantel to limit the prevalence and intensity of hookworm infection, the drug may limit morbidity and be more cost-effective than anticipated, based on expected efficacy against schistosomiasis alone.
Footnotes
Authors' addresses: Julia G. Shaw, Master of Public Health Program, Department of Community Health and Center for International Health Research, Rhode Island Hospital, Brown University School of Medicine, Providence, RI, E-mail: Julia_G_Shaw@brown.edu. Nitin Aggarwal, Center for International Health Research, Departments of Pediatrics and Pathology, and Laboratory Medicine, Rhode Island Hospital, Brown University School of Medicine, Providence, RI, E-mail: Nitin_Aggarwal@brown.edu. Luz P. Acosta, Research Institute for Tropical Medicine, Manila, the Philippines, E-mail: LPAcosta@yahoo.com. Mario A. Jiz, Center for International Health Research and Department of Pathology and Laboratory Medicine, Rhode Island Hospital, Brown University School of Medicine, Providence, RI and Research Institute for Tropical Medicine, Manila, the Philippines, E-mail: Mario.a.jiz@gmail.com. Hai-Wei Wu, Tjalling Leenstra, and Jennifer F. Friedman, Center for International Health Research and Department of Pediatrics, Rhode Island Hospital, Brown University School of Medicine, Providence, RI, E-mails: Haiwei_Wu@brown.edu, T.leenstra@hotnet.nl, and jennifer_friedman@brown.edu. Hannah M. Coutinho, Center for International Health Research, Departments of Pediatrics and Pathology, and Laboratory Medicine, Rhode Island Hospital, Brown University School of Medicine, Providence, RI, E-mail: H.m.coutinho@hotmail.com. Remigio M. Olveda, Research Institute for Tropical Medicine, Manila, the Philippines, E-mail: Remi_olveda@yahoo.com.ph. Jonathan D. Kurtis, Center for International Health Research and Department of Pathology and Laboratory Medicine, Rhode Island Hospital, Brown University School of Medicine, Providence, RI, E-mail: Jonathan_Kurtis@brown.edu. Stephen T. McGarvey, Epidemiology Section, Department of Community Health and International Health Institute, Brown University, Providence, RI, E-mail: Stephen_McGarvey@brown.edu.
References
- 1.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. J Am Med Assoc. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 4.Utzinger J, Vounatsou P, N'Goran EK, Tanner M, Booth M. Reduction in the prevalence and intensity of hookworm infections after praziquantel treatment for schistosomiasis infection. Int J Parasitol. 2002;32:759–765. doi: 10.1016/s0020-7519(02)00012-7. [DOI] [PubMed] [Google Scholar]
- 5.Montero R, Ostrosky P. Genotoxic activity of praziquantel. Mutat Res. 1997;387:123–139. doi: 10.1016/s1383-5742(97)00027-6. [DOI] [PubMed] [Google Scholar]
- 6.Blair KL, Bennett JL, Pax RA. Praziquantel: physiological evidence for its site(s) of action in magnesium-paralysed Schistosoma mansoni. Parasitology. 1992;104:59–66. doi: 10.1017/s0031182000060807. [DOI] [PubMed] [Google Scholar]
- 7.Hall TM, Joseph GT, Strand M. Schistosoma mansoni: molecular cloning and sequencing of the 200-kDa chemotherapeutic target antigen. Exp Parasitol. 1995;80:242–249. doi: 10.1006/expr.1995.1030. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Preventive Chemotherapy in Human Helminthiasis. Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 9.Buck AA, Anderson RI, MacRae AA. Epidemiology of poly-parasitism. IV. Combined effects on the state of health. Tropenmed Parasitol. 1978;29:253–268. [PubMed] [Google Scholar]
- 10.Chunge RN, Karumba N, Ouma JH, Thiongo FW, Sturrock RF, Butterworth AE. Polyparasitism in two rural communities with endemic Schistosoma mansoni infection in Machakos District, Kenya. J Trop Med Hyg. 1995;98:440–444. [PubMed] [Google Scholar]
- 11.Keiser J, N'Goran EK, Singer BH, Lengeler C, Tanner M, Utzinger J. Association between Schistosoma mansoni and hookworm infections among schoolchildren in Cote d'Ivoire. Acta Trop. 2002;84:31–41. doi: 10.1016/s0001-706x(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 12.Tchuem Tchuente LA, Behnke JM, Gilbert FS, Southgate VR, Vercruysse J. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Trop Med Int Health. 2003;8:975–986. doi: 10.1046/j.1360-2276.2003.01120.x. [DOI] [PubMed] [Google Scholar]
- 13.Brooker S, Clements AC. Spatial heterogeneity of parasite co-infection: determinants and geostatistical prediction at regional scales. Int J Parasitol. 2009;39:591–597. doi: 10.1016/j.ijpara.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezeamama AE, McGarvey ST, Acosta LP, Zierler S, Manalo DL, Wu HW, Kurtis JD, Mor V, Olveda RM, Friedman JF. The synergistic effect of concomitant schistosomiasis, hookworm, and Trichuris infections on children's anemia burden. PLoS Negl Trop Dis. 2008;2:e245. doi: 10.1371/journal.pntd.0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGarvey ST, Aligui G, Graham KK, Peters P, Olds GR, Olveda R. Schistosomiasis japonica and childhood nutritional status in northeastern Leyte, the Philippines: a randomized trial of praziquantel versus placebo. Am J Trop Med Hyg. 1996;54:498–502. doi: 10.4269/ajtmh.1996.54.498. [DOI] [PubMed] [Google Scholar]
- 16.Leenstra T, Acosta LP, Langdon GC, Manalo DL, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines. Am J Clin Nutr. 2006;83:371–379. doi: 10.1093/ajcn/83.2.371. [DOI] [PubMed] [Google Scholar]
- 17.Friedman JF, Kanzaria HK, Acosta LP, Langdon GC, Manalo DL, Wu H, Olveda RM, McGarvey ST, Kurtis JD. Relationship between Schistosoma japonicum and nutritional status among children and young adults in Leyte, the Philippines. Am J Trop Med Hyg. 2005;72:527–533. [PubMed] [Google Scholar]
- 18.Koukounari A, Fenwick A, Whawell S, Kabatereine NB, Kazibwe F, Tukahebwa EM, Stothard JR, Donnelly CA, Webster JP. Morbidity indicators of Schistosoma mansoni: relationship between infection and anemia in Ugandan schoolchildren before and after praziquantel and albendazole chemotherapy. Am J Trop Med Hyg. 2006;75:278–286. [PubMed] [Google Scholar]
- 19.Albonico M, Savioli L. Hookworm infection and disease: advances for control. Ann Ist Super Sanita. 1997;33:567–579. [PubMed] [Google Scholar]
- 20.World Health Organization . Prevention and Control of Schistosomiasis and Soil Transmitted Helminthiasis. Geneva: World Health Organization; 2002. pp. 1–57. [PubMed] [Google Scholar]
- 21.Widjana DP, Sutisna P. Prevalence of soil-transmitted helminth infections in the rural population of Bali, Indonesia. Southeast Asian J Trop Med Public Health. 2000;31:454–459. [PubMed] [Google Scholar]
- 22.Tarafder MR, Carabin H, Joseph L, Balolong E, Jr, Olveda R, McGarvey ST. Estimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a ‘gold standard.’. Int J Parasitol. 2009;40:399–404. doi: 10.1016/j.ijpara.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmann P, Du ZW, Wang LB, Wang XZ, Jiang JY, Li LH, Marti H, Zhou XN, Utzinger J. Extensive multiparasitism in a village of Yunnan province, People's Republic of China, revealed by a suite of diagnostic methods. Am J Trop Med Hyg. 2008;78:760–769. [PubMed] [Google Scholar]
- 24.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, Marti H, Utzinger J. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2:e331. doi: 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leenstra T, Coutinho HM, Acosta LP, Langdon GC, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Schistosoma japonicum reinfection after praziquantel treatment causes anemia associated with inflammation. Infect Immun. 2006;74:6398–6407. doi: 10.1128/IAI.00757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ezeamama AE, Friedman JF, Olveda RM, Acosta LP, Kurtis JD, Mor V, McGarvey ST. Functional significance of low-intensity polyparasite helminth infections in anemia. J Infect Dis. 2005;192:2160–2170. doi: 10.1086/498219. [DOI] [PubMed] [Google Scholar]
- 27.Stothard JR. Improving control of African schistosomiasis: towards effective use of rapid diagnostic tests within an appropriate disease surveillance model. Trans R Soc Trop Med Hyg. 2009;103:325–332. doi: 10.1016/j.trstmh.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Olds GR, King C, Hewlett J, Olveda R, Wu G, Ouma J, Peters P, McGarvey S, Odhiambo O, Koech D, Liu CY, Aligui G, Gachihi G, Kombe Y, Parraga I, Ramirez B, Whalen C, Horton RJ, Reeve P. Double-blind placebo-controlled study of concurrent administration of albendazole and praziquantel in schoolchildren with schistosomiasis and geohelminths. J Infect Dis. 1999;179:996–1003. doi: 10.1086/314686. [DOI] [PubMed] [Google Scholar]
- 29.Utzinger J, Rinaldi L, Lohourignon LK, Rohner F, Zimmermann MB, Tschannen AB, N'Goran EK, Cringoli G. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg. 2008;102:84–90. doi: 10.1016/j.trstmh.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Booth M, Vounatsou P, N'Goran EK, Tanner M, Utzinger J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Cote d'Ivoire. Parasitology. 2003;127:525–531. doi: 10.1017/s0031182003004128. [DOI] [PubMed] [Google Scholar]


