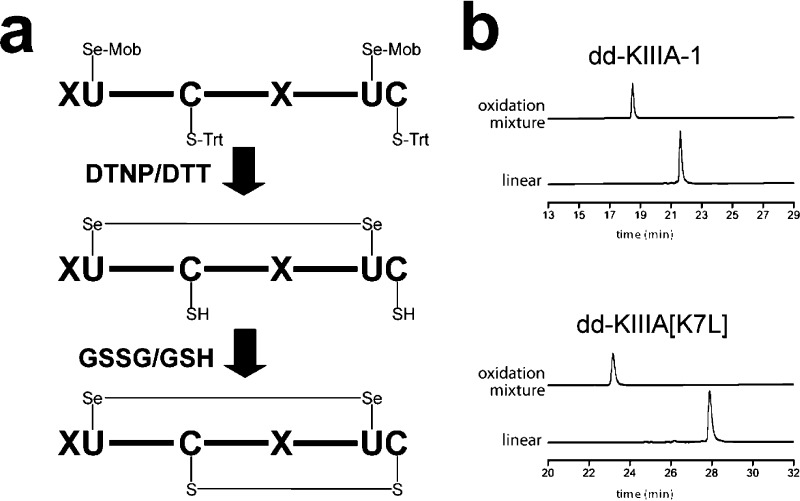
Figure 3.
Chemical synthesis and oxidative folding of the disulfide-depleted selenopeptide analogues of KIIIA. (a) An overview of the chemical synthesis strategy for ddKIIIA analogues. The selenopeptide analogue is removed from a resin by the treatment with an enriched reagent K (DTNP removes Mob-protecting groups from Sec residues, whereas subsequent treatment with DTT removes the resulting 5-thionitropyridyl derivatives by thiolysis). The redox-favored diselenide bridge is formed at low pH, and its presence was confirmed by mass spectrometry and alkylation analyses (Figure S1 in the Supporting Information). The formation of the remaining single disulfide bridge is promoted by oxidants, such as glutathione. (b) HPLC elution profiles of the oxidative folding of two ddKIIIA analogues. The HPLC-purified linear form contains preexisting diselenide bridge, imposing only one possible oxidation product, as confirmed by HPLC analyses of the oxidation mixtures.