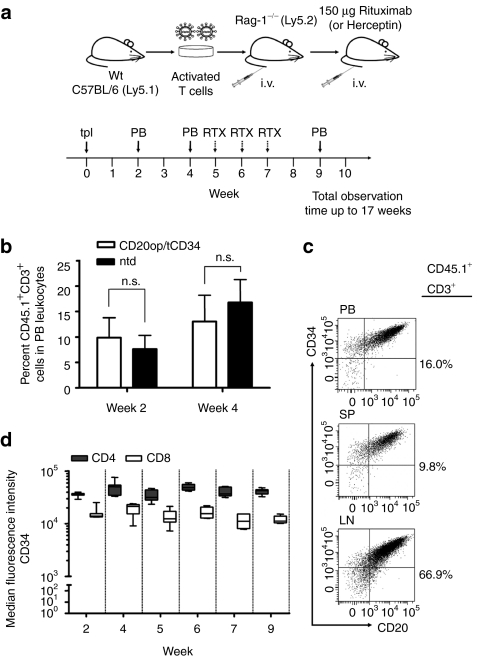
Figure 3.
Experimental design and repopulation of Rag-1-deficient mice with CD20op/tCD34-transduced T cells. (a) Mature T cells were isolated from C57BL/6.Ly5.1 mice, stimulated with anti-CD3/CD28 beads, and 100 U/ml interleukin 2, and transduced 72 and 96 hours after stimulation. At day 7, vector-transduced T cells were immunoselected using anti-CD34 beads and transplanted into Rag-1-deficient recipient mice (indicated by solid arrow at week 0). Blood was collected from recipient mice 2 and 4 weeks after transplantation [peripheral blood (PB), indicated by solid arrows] and analyzed by flow cytometry for the presence of engrafted CD45.1+CD3+ donor T cells as well as for CD20op and tCD34 surface expression in transduced cells. At week 5, 6, and 7 mice received 150 µg Rituximab intravenously for cell depletion (indicated by dashed arrows). Herceptin was used as a negative control. Two days after each injection, blood was analyzed by flow cytometry. Animals were observed for up to 17 weeks. (b) Repopulation of Rag-1-deficient mice with vector-transduced, immunoselected (white bars; n = 20) and nontransduced (black bars; n = 9) donor T cells. Blood was collected at weeks 2 and 4 after adoptive transfer and analyzed for CD45.1+CD3+ donor T cells. Analysis gate was set on PB leukocytes and shown are the mean percentages of CD45.1+CD3+ donor cells ± SD from two independent experiments (n.s., not significant, P > 0.05). (c) Co-expression of CD20op and tCD34 in murine T cells. CD3+ cells obtained at week 17 from peripheral blood, spleen (SP), and lymph nodes (LNs) of control animals were analyzed for CD20op and tCD34 surface expression. Analysis gate was set on CD45.1+CD3+ leukocytes and scatter plots show the expression of CD20op and tCD34 in PB donor cells and T cells isolated from SP and LNs from one representative animal. Numbers next to each scatter plot indicate the percentage of CD45.1+CD3+ cells. (d) Stability of tCD34 expression levels in adoptively transferred T cells in Rag-1-deficient mice. Peripheral blood was collected at the indicated time points and stained for tCD34 expression in CD4 donor T cells. Analysis gate was set on CD45.1+CD3+ donor cells in peripheral blood leukocytes. The box–whisker plot indicates the median fluorescence intensity of CD34 staining in both CD4 (gray boxes) and CD8 (white boxes) subsets from 7 animals.