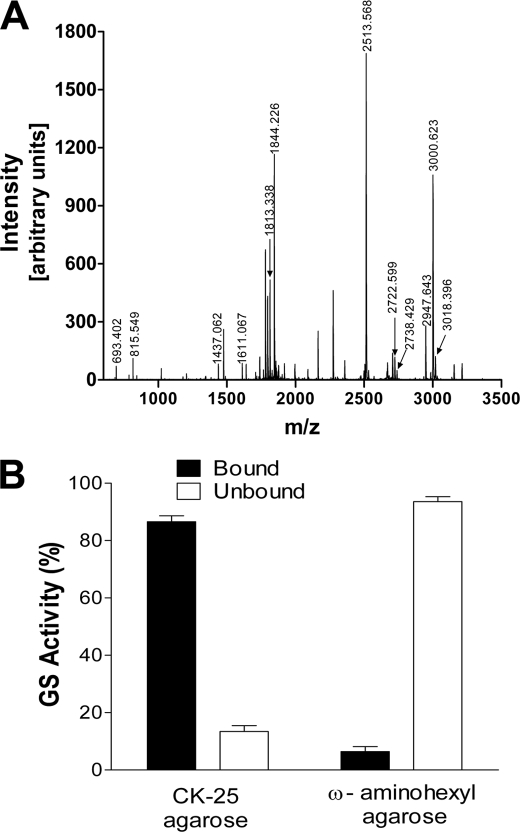
FIGURE 2.
Identification of the 40-kDa protein as soybean cytosolic glutamine synthetase. A, MALDI-TOF spectrum of protonated tryptic peptides (MH+) of the 40-kDa protein isolated by affinity chromatography on nod26 peptide-agarose. The 40-kDa protein was resolved by electrophoresis as in Fig. 1B and subjected to in-gel tryptic digestion and mass spectroscopic analysis. The y axis shows the intensity as arbitrary units. The mass-to-charge ratio is plotted on the x axis. The results of mass fingerprinting analysis are in Table 1. B, interaction of GS with the C-terminal domain of nod26. Purified native soybean nodule GS (49 units) was incubated with nod26 peptide-agarose (CK-25-agarose) or with underivatized ω-aminohexyl-agarose. The fraction of GS activity bound to the resins (solid bars) as well as in the unadsorbed fraction (open bars) was measured (n = 6, error bars show S.E.).