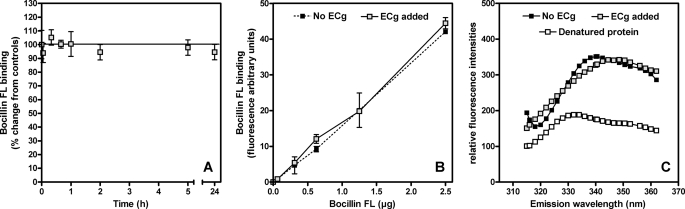
FIGURE 5.
β-Lactam binding (Aand B) and fluorescence assay (C) of PBP2a in 25 mm HEPES, 1 m NaCl (pH 7. 0). A, binding of the fluorescent penicillin derivative Bocillin FL to PBP2a (5 μm). PBP2a was incubated for up to 24 h in 12.5 μg/ml ECg. The samples were then incubated for 25 min in the presence of 1.25 μg of Bocillin FL and prepared for fluorescence measurement. The results were expressed as percentages of change from controls (no ECg added). The values are the means ± S.D. B, binding of increasing concentrations of Bocillin FL to PBP2a (5 μm). PBP2a was incubated over 1 h in 12.5 μg/ml ECg. Binding was then measured after 25 min in the presence of increasing concentrations of Bocillin FL. The values are the means ± S.D. C, tryptophan fluorescence emission spectra of PBP2a (0.18 μg/ml). The pretreatments of PBP2a are as follows: ECg added (1 h, 12.5 μg/ml) and denatured protein (1 h, 60 °C). We have previously determined (36) that the Bocillin FL concentrations employed in these experiments enable the detection of changes in the affinity of PBP2a for β-lactam antibiotics.