FIGURE 7.
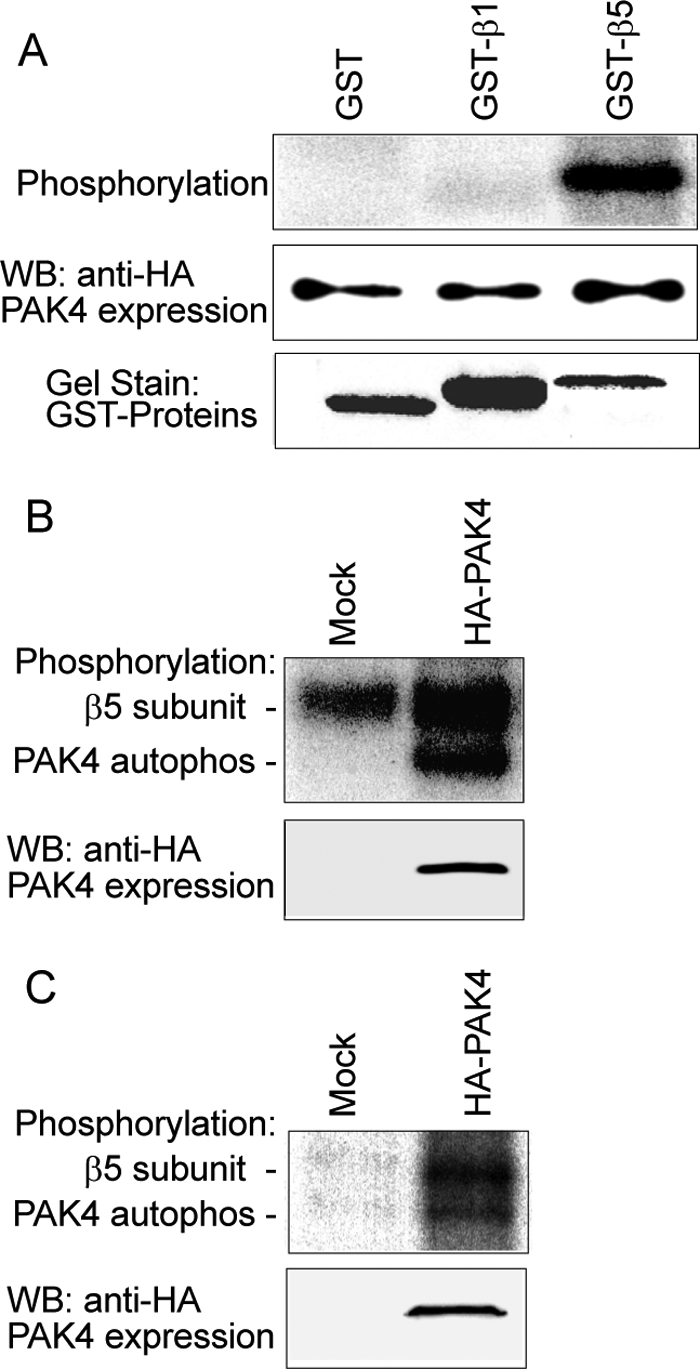
PAK4 phosphorylates the integrin β5 subunit. PAK4 was immunoprecipitated using an anti-HA mAb from COS-7 cells transfected with an HA-PAK4 vector and incubated with integrins in the presence of [γ-32P]ATP. A, PAK4 phosphorylation of integrin β5 cytoplasmic domain analyzed by in vitro phosphorylation using purified GST, GST-β1, or GST-β5 cytoplasmic domain as substrates. PAK4 levels detected by immunoblot (middle panel) and the amounts of GST fusion proteins used are indicated by staining with Coomassie Brilliant Blue (lower panel). B, in the same manner, 5 μg of purified integrin αvβ5 was analyzed for phosphorylation by immunoprecipitated PAK4, separated by 7.5% SDS-PAGE, and visualized by autoradiography. C, PAK4 phosphorylates β5 subunit in living cells. Cells underwent phosphate starvation and then metabolic labeling as described under “Experimental Procedures.” Integrin αvβ5 was immunoprecipitated in cells with or without overexpressed HA-PAK4, exposed to SDS-PAGE and autoradiography (upper panel). The lower panel shows the immunoblot for HA-PAK4 expression.
