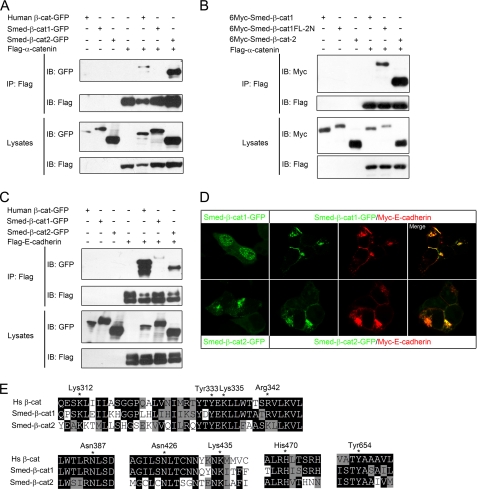
FIGURE 2.
Smed-β-catenin-2 interacts with the cadherin complex. A, HEK293T cells were transfected with the indicated plasmids, and lysates were immunoprecipitated (IP) with FLAG-M2 beads. Western blotting (IB) with anti-GFP revealed co-IP of Smed-β-catenin-2 but not Smed-β-catenin-1 following FLAG-M2 immunoprecipitation, indicating that only Smed-β-catenin-2 interacts with α-catenin. B, co-IP experiments in HEK293T cells indicated that the transplantation of the α-catenin-binding domain into Smed-β-catenin-1 rescued its interaction with α-catenin. C, co-IP with E-cadherin reveals an interaction with both Smed-β-catenin-1 and -2. D, localization of Smed-β-catenins alone or co-expressed with E-cadherin in transfected HEK293T cells. E, alignments of stretches of amino acid sequences from human β-catenin and Smed-β-catenin-1 and -2 that are important for β-catenin/cadherin interaction. Essential residues for the interaction are indicated by stars and numbered in human β-catenin.