Figure 7.
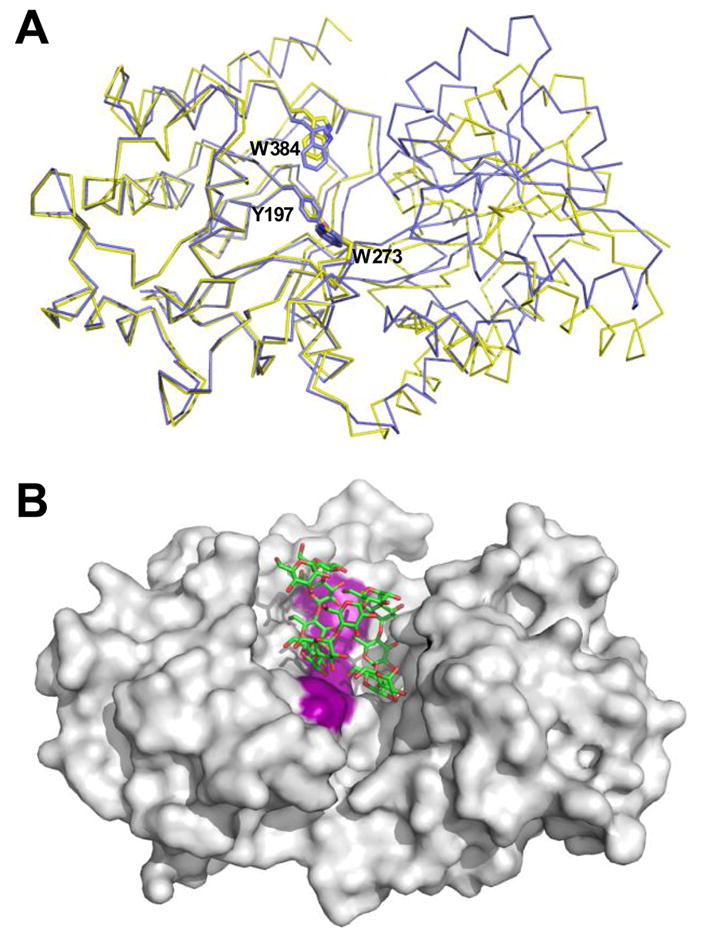
Structural changes and ligand accommodation in MalX. A) Overlay of MalX in the maltoheptaose complexed form (shown as a blue Cα trace) and the apo form (yellow) made by overlapping only the large C-terminal domain. The amino acids making up the aromatic binding platform are shown in stick representation and labelled. B) solvent accessible surface of the apo form of MalX with an idealized maltododecaose molecule (green sticks) modelled into the active site. The aromatic binding platform is colored purple for reference.
