Figure 6.
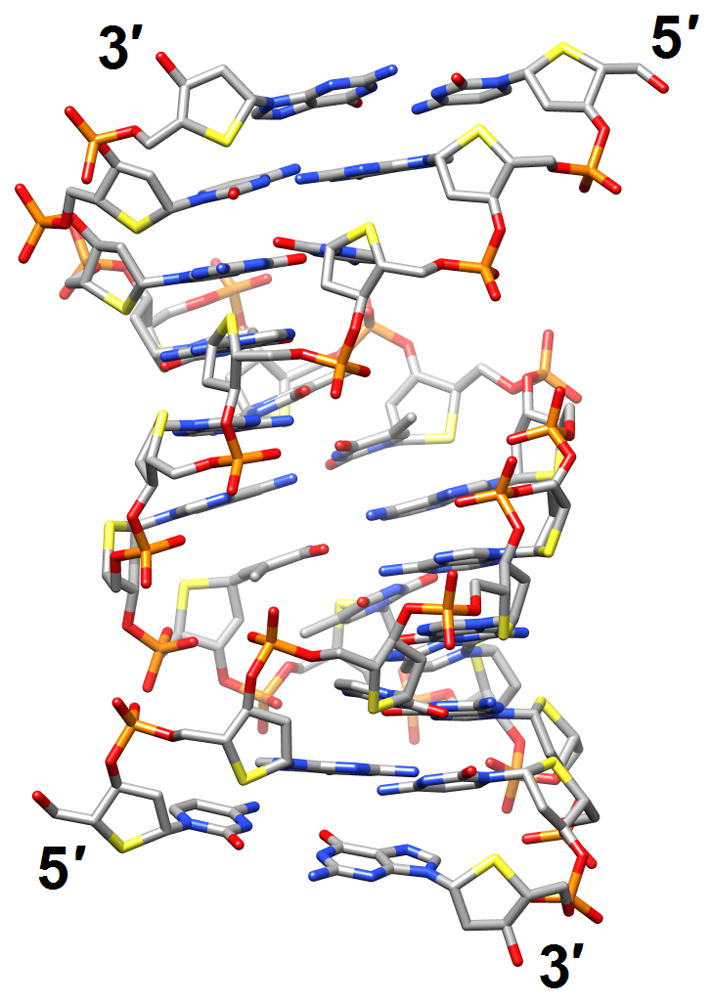
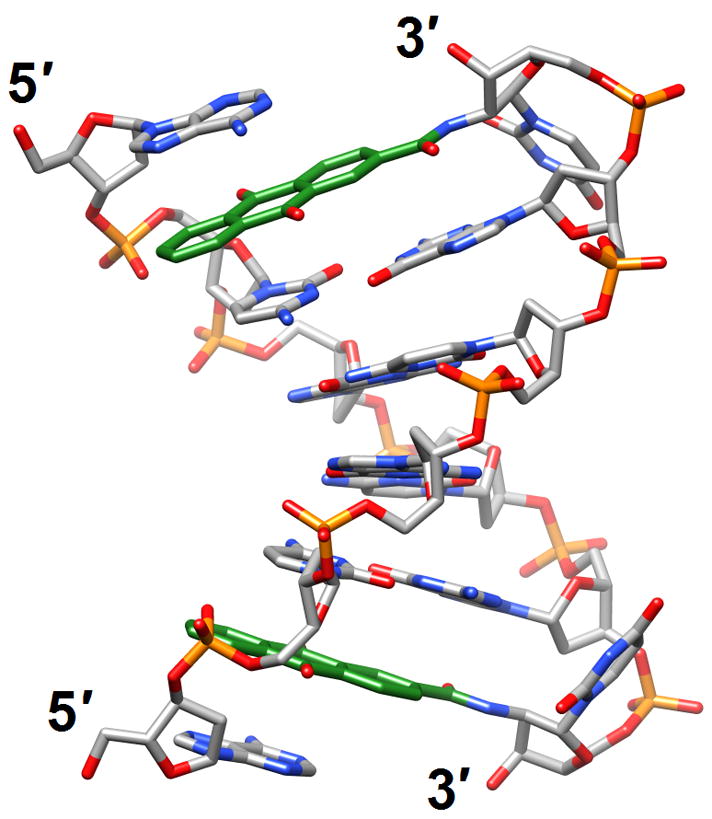
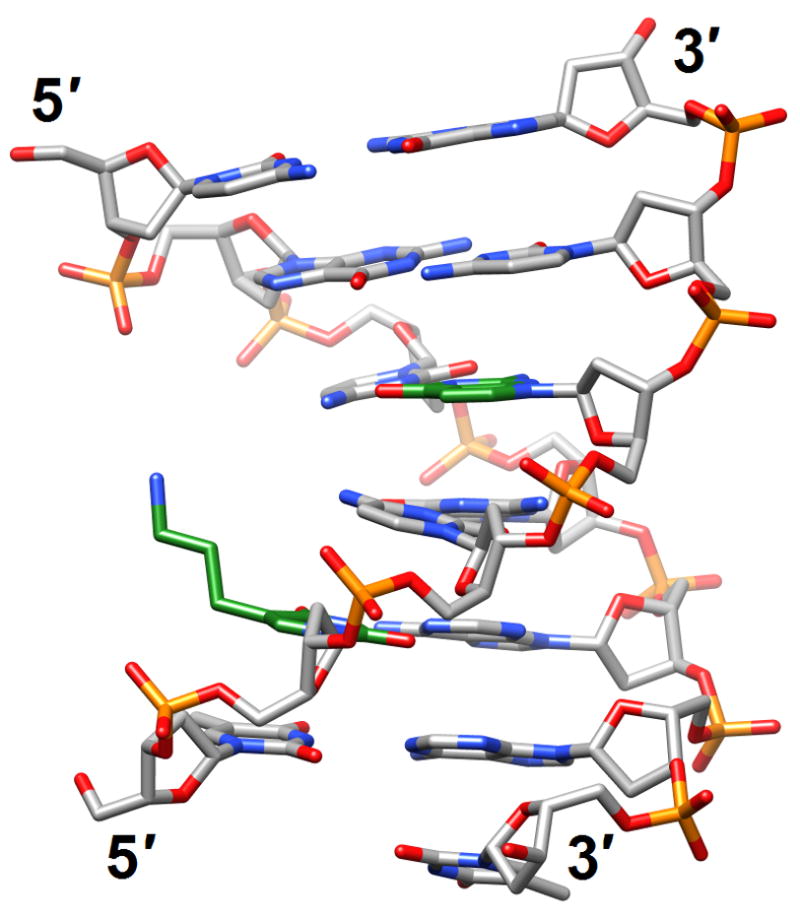
Structural consequences of chemical modification. (a) A-form geometry of the duplex formed by 4′-thio-DNA [65•] (2rmq). Sulfur atoms are highlighted in yellow. (b) Extensive stacking interactions and hydrogen bonding are at the origin of the dramatic stability increases with DNA and RNA duplexes capped by the 3′-terminal 2′-anthraquinoylamido-2′-deoxyuridine residue [71] (2kk5). Carbon atoms of the aromatic moiety are highlighted in green. (c) Orientation of the tethered cation from 5-(3-aminopropyl)-2′-dU8 relative to 7-deaza-G10 (N+2) in the NMR structure of a modified DDD [72] (2qef). Carbon atoms of 5-(3-aminopropyl)-uracil and 7-deaza-guanine are highlighted in green.
