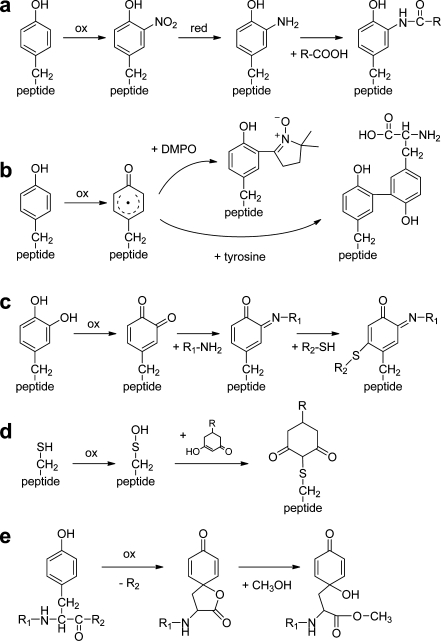
Fig. 4.
Secondary labeling reactions of oxidized amino acid residues. a Oxidative nitration, followed by reduction of the nitro group to an amine, and finally amide formation [27, 131]. b Reaction of the radical intermediate of Tyr with the spin-label 5,5-dimethylpyrroline-N-oxide (DMPO) [134] or a second Tyr residue [51]. c Secondary oxidation of hydroxylated Tyr to an orthoquinone, followed by reaction with an amine (e.g., a protein Lys), leading to a Schiff base intermediate, which is very reactive towards thiols [51]. d Reaction of sulfenic acid with a linker molecule containing secondary amine or azide functional groups for labeling [137]. e Tyr peptide bond cleavage leads to a lactone intermediate that can react with alcohols or amines [138]