Abstract
Ghrelin is a 28-amino-acid peptide that displays a strong growth hormone- (GH-) releasing activity through the activation of the growth hormone secretagogue receptor (GHSR). The first studies about role of ghrelin were focused on its orexigenic ability, but despite indisputable pharmacological data, the evidence for a physiological role for ghrelin in the control of appetite is much less clear. Mice with targeted deletion of either ghrelin or the GHSR exhibit an essentially normal metabolic phenotype when fed a regular chow diet, suggesting that ghrelin may have a redundant role in the regulation of food intake. RNAs for ghrelin as well as GHSR are expressed in the pancreas of rats and humans and several studies propose that ghrelin could have an important function in glucose homeostasis and insulin release, independent of GH secretion. Low plasma ghrelin levels are associated with elevated fasting insulin levels and insulin resistance, suggesting both physiological and pathophysiological roles for ghrelin. For this reason, at least theoretically, ghrelin and/or its signalling manipulation could be useful for the treatment or prevention of diseases of glucose homeostasis such as type 2 diabetes.
1. Introduction
GH is released from the pituitary gland in a pulsatile manner and it is mainly regulated by episodic changes in two hypothalamic hormones, growth hormone-releasing hormone (GHRH) and somatostatin. GHRH stimulates GH secretion whereas that somatostatin inhibits it [1]. In 1976, it was revealed that modified opioid peptides had low GH secretory activity [2]. Since then, many efforts have been made to develop and improve potential applications of these GH secretagogues (GHSs) [3–7]. GHSs act on the pituitary and hypothalamus to release GH, not through the growth hormone releasing hormone receptor (GHRHR) but through an orphan receptor, the GHSR [8]. These facts indicated that an unknown endogenous ligand for GHSR should exist. In 1999, ghrelin was identified as the endogenous ligand for the GHSR. It is a 28-amino-acid peptide predominantly produced by the stomach that functions as a somatotrophic and orexigenic signal from the stomach [9, 10]. Ghrelin is most abundantly expressed in specialized cells in the oxyntic glands of the gastric epithelium, originally termed X/A-like cells [11]. Approximately 60%–70% of circulating ghrelin is secreted by the stomach, and most of the remainder originates in the small intestine [11]. Nevertheless low-level ghrelin expression also occurs in several tissues outside the gut, including hypothalamus (arcuate nucleus and paraventricular nucleus), pituitary, lung, adrenal cortex, kidney, bone, testis, placenta, and pancreatic islet cells [12]. The GHSR mRNA is expressed as two splice variants encoding the cognate receptor GHSR1a and the apparently nonfunctional receptor GHSR1b [13]. GHSR1a signals via inositol trisphosphate (IP3) generation and Ca2+ release and has constitutive activity [13, 14]. GHSR1b mRNA is as widely expressed as ghrelin, whereas GHSR1a gene expression is concentrated in the hypothalamus-pituitary unit, although it is also distributed in other central and peripheral tissues [15]. Ghrelin circulates in the bloodstream in two different forms: acylated (or n-octanoylated, AG) and unacylated (or des-octanoylated or des-acylated, UAG) [9]. AG has a unique feature: a posttranslational esterification of a fatty (n-octanoic or, to a lesser extent, n-decanoic) acid on serine residue at position 3 [9]. Recent data showed that Ghrelin O-acyltransferase (GOAT), a membrane-bound enzyme, is responsible for octanoylation of the serine-3 residue of ghrelin [16, 17]. Ghrelin acylation is considered necessary for its actions via GHSR1a, such as its strong GH-releasing activity [9, 18–20]. Normally AG accounts for less than 10% of the total ghrelin in the circulation. The majority of circulating ghrelin is UAG, which does not have effects in GH release, but it is not biologically inactive [19, 21–29]. It binds with high affinity to a receptor, different from GHSR1a and yet unknown [9, 12]. The first studies about ghrelin demonstrated that it increases food intake and adiposity [10]. Moreover, plasma ghrelin levels have been shown to increase prior a meal and during fasting and to decrease after a meal, and they are negatively correlated with body weight [30–32]. All these data suggested a role in the control of energy homeostasis. But the conflicting food intake and body weight data from transgenic and knockout models, which present normal metabolic phenotype, has made difficult defining a key role for endogenous ghrelin in the control of appetite [27, 33–39]. Nevertheless, the data consistently suggest that ghrelin may be important in the control of glucose homeostasis and insulin release.
It was reported that prolonged treatment with GHSs provoked hyperglycemia and hyperinsulinism but this effect was supposed to reflect increased GH secretion [40–43], as GH plays an important role modulating energy homeostasis and metabolism [44]. Particularly, GH exerts both acute and chronic effects on carbohydrate and lipid metabolism [44]. Interestingly, both actions display an opposite pattern, with acute effects showing a transient “insulin-like” action and chronic effects exhibiting an “anti-insulin” action. In this sense, GH administration decreases blood glucose concentration, stimulates glucose uptake by skeletal muscle, and stimulates glucose transport and lipogenesis in isolated adipocyte [44, 45]. However these effects are transitory; after a few hours the chronic anti-insulin effects of GH arise, increase blood glucose concentration, insulin resistance, stimulation of lypolysis, and inhibition of glucose transport. High plasma GH levels induce hyperinsulinemia and insulin resistance [12, 46]. On the other hand, besides its lipolytic action, GH exhibit antilipogenic effects [47, 48] promoting proliferation of lean tissues, while reducing accumulation of fat tissue. Accordingly, GH-deficient states in humans and rodents are characterized by a decrease in lean body mass accompanied by increased adipose tissue [49–53].
Early studies demonstrated that RNAs for ghrelin, as well as GHSR, are expressed in the pancreas of rats and humans [9, 15, 54, 55] and β-cells lines [56, 57] suggesting a possible relation between ghrelin and insulin. Studies using different experimental systems localized ghrelin-immunoreactive cells in rat and human pancreas in the α-cells [54, 57, 58], β-cells [55], PP-cells [57], and other islets cells [57, 59], including those named ε-cells [60]. The first evidences about an interaction between ghrelin and glucose metabolism arose when it was seen that single subcutaneous ghrelin injections induced an increase of the respiratory quotient (RQ), which suggested an augmented utilization of carbohydrate and reduced utilization of fat to meet energy requirements that was congruent with the observed increase in body fat [10]. Another evidence that suggested that ghrelin could affect glucose metabolism was the fact that it stimulated acid secretion through vagal mediation [61, 62] and some studies suggested that the parasympathetic nerves that regulate hormonal control of insulin pass through the cervical vagus and the hepatic branch, and that the hepatic vagus nerve is important for the regulation of hepatic glucose production in the post absorptive state [63, 64]. All these data and numerous studies since 2000 to the present time suggest that ghrelin has an important role in regulating β-cell function and glucose homeostasis. Indeed, the weight of evidences could support even a more physiologically important function in the control of glucose homeostasis than appetite regulation.
In this work we will review the results obtained by different investigators about the relation between ghrelin and glucose metabolism and insulin release as well as its possible therapeutic role in disease states like diabetes.
2. Effects of Exogenous Ghrelin on Glucose and Insulin Levels
2.1. Short-Term Effects
The first studies with ghrelin showed that acute ghrelin treatment induced hyperglycemia and reduced insulin secretion in healthy humans [65] during the first hours of treatment. The time course of glucose modifications occurred with a peak observed before any significant insulin decreased. Subsequently, these findings were confirmed by other authors in human and rodents [58, 66–71]. However, when these experiments were carried out in obese patients, there was no difference in glucose or insulin levels following ghrelin administration [72]. The first hypothesis suggested that ghrelin itself could have a direct effect on glucose metabolism, regulating hepatic glucose output, promoting glycogen breakdown, or decreasing peripheral glucose uptake; consistent with this view were the findings that ghrelin receptors are expressed in normal human liver [73, 74].
In order to discard a secondary effect due to increased GH secretion, human subjects were treated with a GH receptor blockade, pegvisomant [43], and in this situation a ghrelin mimetic induced increases in glucose and insulin levels. Suggesting ghrelin mimetic-mediate GH-independent insulin resistance, moreover several studies have demonstrated that interference with ghrelin signalling by use of GHSR antagonist decreases blood glucose in wild-type mice as well as GH-deficient lit/lit mice [58, 75]. To diminish the influence of GH, Vestergaard et al. investigate the effects of prolonged ghrelin infusion (not a unique dose) on insulin sensitivity [76] which decreased after few minutes of ghrelin infusion and outlasted both the infusion period and the postinfusion interval. As the reduced insulin sensitivity remained after normalization of both GH and glucose levels, this work supports that ghrelin effect was caused by the ghrelin infusion per se [77]. In a posterior work the same investigators studied, for the first time, the concomitant effects of exogenous ghrelin and a pancreatic clamp on glucose metabolism in humans; they used a prolonged ghrelin infusion in addition to a somatostatin infusion to avoid GH secretion. Ghrelin infusion decreased basal as well as insulin stimulated glucose disposal and induced peripheral insulin resistance but did not affect hepatic glucose production [71]. When they studied the effect of exogenous ghrelin in hypopituitary men (in the absence of GH and cortisol secretion), in a randomized double-blind, cross-over design, ghrelin treatment acutely decreased peripheral, but not hepatic, insulin sensitivity independently of GH and cortisol [78].
There are data that suggest a relation between ghrelin and glucose-stimulated insulin secretion (GSIS) via the hepatic portal system and the vagus nerve. Gastrectomy and truncal vagotomy are operations characterized by hypoghrelinemia [30], glucose intolerance as a result of hyperglucagonemia, insulinopenia, and impaired first phase of insulin secretion [79]. When ghrelin was infused into the portal vein of rats, inhibited glucose-stimulated release of insulin, however when it was infused into the femoral vein, did not induce such an inhibitory effect. All the more hepatic vagotomy or coinfusion with atropine methyl bromide (a muscarinic antagonist) diminished the inhibitory effect of ghrelin on glucose-stimulated insulin secretion [70]. Damjanovic et al. also performed studies with ghrelin and truncal vagotomy, investigating the effects of intravenous (IV) ghrelin infusion on insulin-mediated glucose disposal during a hyperinsulinemic-euglycemic clamp in humans who underwent total gastrectomy and truncal vagotomy [80]. In these patients glucose disposal rate (GDR) decreased during ghrelin infusion; however this difference was not translated into a significant difference in insulin concentration, probably because the exogenous insulin by far overweighs endogenous insulin. Thus, there cannot be ruled the possibility that diminished glucose utilization after ghrelin administration is partly explained by the decrease in endogenous insulin secretion, although this was not detected in the study [80]. It appears that acute ghrelin administration might be involved in the negative control of insulin secretion and glucose consumption in gastrectomized patients [80].
In summary, short-term effects of exogenous ghrelin induces hyperglycaemia and hypoinsulinism in health humans and rodents in a GH independent fashion.
In Table 1 are summarized the results obtained after acute ghrelin treatment in several models and situations.
Table 1.
Acute effects of ghrelin administration on glucose-insulin homeostasis in different species and metabolic situations. IV: intravenous; O: oral.
| Species | Treatment | Dose | Food before experiment | Treatment duration | Plasma glucose or GIR | Plasma insulin | Reference |
|---|---|---|---|---|---|---|---|
| Health humans | 1 IV AG injection versus 1 IV placebo injection | 1 μg AG/kg | Fasting overnight | 3 hours | Enhanced | Decreased | [65] |
|
| |||||||
| Health humans | 1 IV AG injection versus 1 IV placebo injection | 3.3 μg AG/kg | Fasting overnight | 3 hours | Enhanced | Decreased | [68] |
|
| |||||||
| Health humans | 1 IV AG injection versus 1 IV placebo injection | 1 μg AG/kg | Fasting overnight | 2 hours | Enhanced | Decreased | [67, 81] |
|
| |||||||
| Health humans | 1 IV AG injection + O-GTT versus O-GTT | 1 μg AG/kg + 100 g glucose | Fasting overnight | 2 hours | Not change | Not change | [81] |
| 1 IV AG injection + FFA versus FFA | 1 μg AG/kg + 10% FFA | Fasting overnight | 2 hours | Not change | Decreased | ||
| 1 IV AG injection + arginine versus arginine | 1 μg AG/kg + 0.5 g arginine/kg | Fasting overnight | 2 hours | Enhanced | Decreased | ||
|
| |||||||
| Health humans | 1 IV AG injection versus 1 IV placebo injection | 1 μg AG/kg | Fasting overnight | 2 hours | Enhanced | Decreased | [67] |
| 1 IV UAG injection versus 1 IV placebo injection | 1 μg UAG/kg | Fasting overnight | 2 hours | Not change | Not change | ||
| 1 IV AG injection + UAG versus 1 IV placebo injection | 1 μg AG/kg + 1 μg UAG/kg | Fasting overnight | 2 hours | Not change | Not change | ||
|
| |||||||
| Health humans | IV AG infusion versus IV placebo infusion | 5 pmol AG/kg/min | Fasting overnight | 3 hours | Enhanced | Enhanced | [77] |
|
| |||||||
| Health humans | IV AG infusion versus IV placebo infusion Both with pancreatic clamp + hyperinsulinemic-euglicemic clamp + glucose adjustable |
5 pmol AG/kg/min | Fasting overnight | 5 hours | During clamp GIR diminished with ghrelin | Not change | [71] |
|
| |||||||
| Hypopituitary humans | 1 IV AG or UAG injection versus 1 IV placebo injection | 1 μg AG or UAG/kg | Fasting overnight | 2 hours | Enhanced | Not change | [82] |
| 1 IV AG + UAG injection versus 1 IV placebo injection | 1 μg AG/kg + 1 μg UAG/kg | Fasting overnight | 2 hours | Not change | Diminished | ||
|
| |||||||
| Hypopituitary humans | IV AG infusion versus IV placebo infusion Both with hyperinsulinemic-euglicemic clamp |
5 pmol AG/kg/min | Fasting overnight | 5 hours | Basal period enhanced, during clamp GIR diminished | Not change | [78] |
|
| |||||||
| Gastrectomized humans | IV AG infusion versus IV placebo infusion Both with hyperinsulinemic-euglycemic clamp |
5 pmol AG/kg/min | Fasting overnight | 5 hours | Diminished GIR | Not change | [80] |
|
| |||||||
| IV AG infusion versus IV placebo infusion Both with IV-GTT infusion |
1 ng AG/kg/h + 13.3 mg glucose/kg/min | 24-hour fasting | 40 minutes | Not change | Not change | ||
| Normal rats | IP AG infusion versus IV placebo infusion Both with IV-GTT infusion |
1 ng AG/kg/h + 13.3 mg glucose/kg/min | 24-hour fasting | 40 minutes | Enhanced | Diminished | [70] |
| IV AG infusion versus IP placebo infusion Both with IP-GTT infusion |
1 ng AG/kg/h + 13.3 mg glucose/kg/min | 24-hour fasting | 40 minutes | Not change | Not change | ||
| IP AG infusion versus IP placebo infusion Both with IP-GTT infusion |
1 ng AG/kg/h + 13.3 mg glucose/kg/min | 24-hour fasting | 40 minutes | Enhanced | Diminished | ||
|
| |||||||
| Normal rats | 1 IV UAG injection + IV-GTT versus IV-GTT | 30 nmol UAG/kg + 1 g glucose/kg | Fasting overnight | 50 minutes | Not change | Enhanced | [83] |
| 1 IV AG injection + IV-GTT versus IV-GTT | 30 nmol UAG/kg + 1 g glucose/kg | Fasting overnight | 50 minutes | Not change | Not change | ||
|
| |||||||
| Rats with hepatic vagotomy | IP AG infusion versus IP placebo infusion Both with IP-GTT infusion |
1 ng AG/kg//h +13.3 mg glucose/kg/min | 24-hour fasting | 40 minutes | Not change | Not change | [70] |
|
| |||||||
| Mice ddY | 1 IP AG injection versus 1 IP placebo injection Both with IP-GTT |
1 and 10 nmol AG/kg + 1 g glucose/kg | Fasting overnight | 2 hours | Enhanced | Decreased | [58] |
| 1 IP AG injection versus 1 IP placebo injection | 1 nmol/kg | Fasting overnight | 2 hours | Enhanced | |||
|
| |||||||
| C57BL/6J mice | 1 IV AG injection + IV-GTT versus IV-GTT | 50 nmol AG/kg + 1g/kg | 3-hour fasting | 50 minutes | Not change | Diminished | [84] |
|
| |||||||
| GH-deficient little mice | 1 IP AG injection versus 1 IP placebo injection | 1 nmol AG/kg | Fasting overnight | 30 minutes | Enhanced | [58] | |
|
| |||||||
| Obese humans | 1 IV AG injection versus IV placebo injection | 1 μg AG/kg | Fasting overnight | 2 hours | Not change | Not change | [72] |
2.2. Long-Term Effects
Generally long-term ghrelin treatment induced an increase in plasmatic values of glucose, whereas plasmatic insulin levels, unlike short-term effects, did not change or enhanced after ghrelin treatment. But long-term effects of exogenous ghrelin on glucose and insulin levels are not conclusive; there are differences inter-experiments which could reflect different doses, administration way, and/or species used. In long term studies essentially there are two way of administration: those in which the administration of ghrelin was systemic: intraperitoneal (IP) or subcutaneous (SC), and central: when the hormone was administered directly in a cerebral region.
2.2.1. Systemic Administration
Involves treated IP with ghrelin during 4 days, plasma glucose concentrations increased. At the same time, the authors measured body glycogen stores and observed that liver glycogen content was unaffected, but the quadriceps muscle and kidney glycogen stores decreased, indicating them as the possible source of elevated plasma glucose levels [69]. Similar results were obtained by Asakawa et al. with mice; they examined the effects of repeated administration of IP ghrelin on glycaemic control under a high fat diet (HFD). In these conditions insulin levels were increased by the treatment and blood glucose concentration displayed a moderate increase but did not reach statistical significance [75].
When Barazzoni et al. administrated subcutaneous ghrelin during four days to normal rats, they found hyperglycemia; nevertheless plasma insulin levels did not change [85, 86]. The treatment increased transcript levels of the key enzyme of the gluconeogenic pathway, glucose-6-phosphatase (G6Pase) in liver. For these reasons the authors suggested that enhanced gluconeogenesis in liver would contribute to increase circulating glucose in ghrelin-treated animals [85].
2.2.2. Central Administration
In others studies the animals received ghrelin treatment intracerebroventricularly (ICV). 6-day ICV ghrelin infusion provoked an increase on insulin-stimulated glucose utilization during euglycemic-hyperinsulinemic clamps in epididymal and inguinal white adipose tissue (WAT) as well as brown adipose tissue (BAT), but not in soleus muscle. During the clamps, hepatic glucose production was comparably suppressed by hyperinsulinemia in all groups. The treatment did not change plasma glucose or insulin levels [87]. Comparable results were obtained by Kamegai et al. administering repeated injections of ghrelin into the lateral ventricle of rats during 72 hours, without changes in plasma glucose and insulin concentrations, although there was a trend toward higher levels [88]. However, in another study, ICV ghrelin injections every 24 hours during five days to adult male rats clearly increased serum insulin levels without evoking changes in blood glucose levels [89].
Although the results obtained by ghrelin treatment in the long term are not enough clear, it seems to exist a tendency toward an increase in both plasma glucose and insulin levels. These data could indicate a role for ghrelin in worsening insulin sensitivity.
In Table 2 are summarized the results obtained in plasma glucose and insulin levels after prolonged treatment with ghrelin.
Table 2.
Chronic effects of ghrelin administration on glucose-insulin homeostasis in different species.
| Species | Treatment administration | Dose | Food during experiment | Duration treatment | Plasma glucose levels | Plasma insulin levels | Reference |
|---|---|---|---|---|---|---|---|
| Mice ddy | 1 IP AG injection/12-h | 3 nmoles AG/mouse/injection | Ad libitum HFD | 5-day | Not change | Enhanced | [75] |
| Tundra vole | 1 IP AG injection/day | 10 μg AG/kg/day | Ad libitum SCD | 4-day | Enhanced | [69] | |
| Sprague-Dawley rats | 1 ICV AG injection/12-h | 1 μg AG/rat/injection | Ad libitum SCD | 3-day | Not change | Not change | [88] |
| Wistar rats | 1 ICV ghrelin injection/day | 1 μg AG/rat/day | Ad libitum SCD | 5-day | Not change | Enhanced | [89] |
| ICV ghrelin infusion | 2.5 nmol AG/rat/day | Ad libitum SCD | 6-day | Not change | Not change | [87] | |
| 1 SC AG injection/12-h | 0.2 ug AG/injection | Ad libitum SCD | 4-day | Enhanced | Not change | [85] |
2.3. Studies In Vitro and Perfusion
Besides the experiments carry out in vivo, there are works with cellular cultures and pancreatic perfusion that contribute to our knowledge about ghrelin role on glucose and insulin metabolism, pointing to a role for ghrelin in the pancreatic islet. The perfused rat pancreas is a suitable model to characterize the pancreatic hormone secretory pattern elicited by ghrelin in the short term. Egido et al. dissected and perfused in situ the pancreas of rats fed ad libitum; the addition of ghrelin to the perfusate did not significantly modify basal insulin release but markedly inhibited the insulin response to increasing glucose concentrations, arginine, and carbachol [90]. It was observed that the glucose-induced insulin release from the rat-perfused pancreas was markedly enhanced by blockade of GHSR and immunoneutralization of endogenous ghrelin. Furthermore, GHSR blockade increased plasma insulin concentrations in gastrectomized and normal rats to a similar extent [91]. The results obtained with perfused rat pancreas support a role for ghrelin inhibiting insulin release. These results were confirmed in studies with isolated islets from normal rats [58, 92] and MIN 6 cells [93], where ghrelin inhibited the insulin response to increasing glucose concentrations. But when ghrelin was coincubated with GHSR antagonists or antiserum against acylated ghrelin, this effect was blocked [58, 92]. Moreover, in islets from ghrelin-null mice, glucose treatment enhanced insulin release [91]. On the contrary, in another study, it was observed that ghrelin (1 pmol/l) stimulated insulin release and increased [Ca2+] in rat islet β-cells in the presence of a stimulatory (8.3 mmol/l) but not basal (2.8 mmol/l) glucose concentration [54]. However, the same authors, in a subsequent study, examined the dose-dependent effects of ghrelin and they found that ghrelin at 1 pmol/l and 0.1 nmol/l modestly potentiated glucose-induced [Ca2+]i responses in a little portion of β-cells, but it failed to significantly alter insulin release. This observation that ghrelin is inhibitory at relatively high concentrations of 10 nmol/l, while having little effect at lower concentrations, is consistent with the majority of other reports [58].
Several cell culture studies showed a genetic link between ghrelin and insulin. The Nkx2.2 hoursomeodomain transcription factor is required for islet cell development and differentiation. In this way high levels of Nkx2.2 are necessary to specify or maintain the islet β cell fate [94]. Nkx2.2 null mice completely lack insulin-producing β-cells and have reduced numbers of α-cells. In normal islets, a population of glucagon-expressing α-cells coexpress ghrelin, but approximately two-thirds of ghrelin-expressing cells define a new endocrine islet, ε cell population. In addition, in the Nkx2.2 mutant islet, the ghrelin-producing ε cell population has been drastically expanded at the expense of insulin- and glucagon-producing cells. [60]. Similar to the wild-type islet, ghrelin producing cells in the Nkx2.2 mutant embryonic mouse islets do not coexpress insulin, somatostatin, or PP. However, unlike its expression in wild-type islets, none of the ghrelin-producing cells in the Nkx2.2 mutant coexpress glucagon [60].
On the other hand, insulinoma-associated protein (IA)-2β is a β-cell autoantigen for type 1 diabetes. It is localized in secretory granules in pancreatic β-cells or neuroendocrine cells [95]. Stable overexpression of IA-2β inhibited GSIS in MIN6 cells when performed in medium containing glucose. Doi et al. observed that ghrelin inhibits GSIS in MIN6 cells and that the concentrations of ghrelin inhibiting GSIS were very close to those of ghrelin enhancing IA-2β expression, suggesting that ghrelin may inhibit GSIS via enhancement of IA-2β expression [93]. Incubation of cultured MIN6 cells with increasing doses/times of ghrelin showed that ghrelin induced IA-2β RNA and protein expression dose dependently. The blockage of IA-2β expression with siRNA provoked that the inhibitory effects of ghrelin or overexpression of IA-2β on GSIS were ameliorated, providing direct evidence of the links between ghrelin, IA-2β, and GSIS; changes in insulin content in the cell lysates or in insulin mRNA expression were not observed [93].
Some of the results obtained with this type of techniques are displayed in Table 3.
Table 3.
Results obtained with cellular cultures and pancreatic perfusion that contribute to data about ghrelin role on glucose and insulin metabolism.
| Cellular type/Perfusion | Treatment | Dose | Insulin release | Glucose output | Reference |
|---|---|---|---|---|---|
| Islets from normal rats | AG + glucose versus glucose | 10−12M AG + 2.8 mM glucose | Not change | [54] | |
| AG + glucose versus glucose | 10−12M AG + 8.3 mM glucose | Enhanced | |||
|
| |||||
| Islets from normal rats | AG + glucose versus glucose | 10−8M AG + 2.8 mM glucose | Not change | [58] | |
| AG + glucose versus glucose | 10−8M AG + 8.3 mM glucose | Diminished | |||
|
| |||||
| Islets from normal rats | AG + glucose versus glucose | 10 nM AG + 20 mM glucose | Diminished | [92] | |
| UAG + glucose versus glucose | 1 μM + 20 mM glucose | Not change | |||
| AG + glucose + YIL-781 versus glucose | 10 nM AG + 20 mM glucose + 1 μM YIL-781 | Not change | |||
| Glucose + YIL-781 versus glucose | 20 mM glucose + 1 μM YIL-781 | Not change | |||
|
| |||||
| Islets from normal rats | GHRP-6 versus placebo | 1 μM GHRP-6 | Enhanced | [58] | |
| SPA versus placebo | 1 μM SPA | Enhanced | |||
|
| |||||
| Ghrelin KO mouse islets | Glucose ghrelin KO versus glucose wildtype | 8.3 mM and 16.7 mM glucose | Enhanced | [91] | |
|
| |||||
| Min 6 cells | AG + glucose versus glucose | 1–10 nM AG + 22.2 mM glucose | Diminished | [93] | |
|
| |||||
| Hepatocytes from pigs | AG versus placebo | 100 nM AG | Enhanced | [96] | |
| UAG versus placebo | 100 nM UAG | Diminished | |||
| UAG + AG versus AG | 100 nM AG + 100 nM UAG | Diminished | |||
|
| |||||
| Pancreas of rat perfused in situ | Ghrelin + glucose versus glucose | 10 nM ghrelin + 5.5 mM glucose | Not change | [90] | |
| Ghrelin + glucose versus glucose | 10 nM ghrelin + 9 mM glucose | Diminished | |||
|
| |||||
| Pancreas of rat perfused in vitro | Ghrelin + glucose versus glucose | 10 nM ghrelin + 8,3 mM glucose | Diminished | [91] | |
| GHRP-6 + glucose versus glucose | 1 μM GHRP-6 + 8.3 mM glucose | Enhanced | |||
| UAG + glucose versus glucose | 10 nmol/l UAG + 8.3 mM glucose | Not change | |||
2.4. Unacylated Ghrelin
Acylated ghrelin accounts for less than 10% of the total ghrelin; the majority of circulating ghrelin is unacylated. Although UAG does not possess GH releasing activity, it is not biologically inactive. Several studies demonstrated a clear metabolic role for UAG; it is able to share with ghrelin antiproliferative effects on human breast and prostate cancer lines [97, 98], has negative inotropic effects on papillary muscle [99], and can stimulate bone marrow adipogenesis [28]. These effects of UAG could not be antagonized by administration of synthetic GHSR1a antagonists [28] as UAG is unable to bind the classical GHSR1a, which recognizes ghrelin in its acylated form only [9]. The signal transduction mechanism(s) for effects of UAG has not been determined. Evidences that UAG is an active peptide implies the existence of GHSR subtypes that recognize and bind ghrelin independently of its acylation. These binding sites have already been demonstrated in the cardiovascular system and in the pancreas [21, 98, 100]. Besides the effects above mentioned, several studies suggested a role of UAG on glucose metabolism. Broglio and colleagues suggested that ghrelin could have a dualistic effect on glucose homeostasis; its effect on insulin secretion and sensitivity could depend on its state of acylation. They observed that in healthy humans, the administration of UAG alone did not induce any change in glucose and insulin levels compared to placebo. Nevertheless UAG counteracts the effects of AG on glucose and insulin levels, but not its stimulatory action on GH, PRL, ACTH, and cortisol levels, indicating that UAG has metabolic impact, being able to antagonize the effects of AG on insulin and glucose levels, while it is really inactive from the neuroendocrine point of view [67].
Similar results were obtained in humans with pituitary insufficiency. In these patients both AG and UAG immediately increase glucose and insulin levels, when AG and UAG were injected together; this combination prevents the acute hyperglycaemic and hyperinsulinemic effects of AG and UAG when injected alone. Moreover, this combination of AG and UAG improves insulin sensitivity for many hours when compared with placebo administration and even more markedly with the aggravation of insulin sensitivity of AG administration [82].
As both AG and UAG are secreted into the portal circulation before they reach the systemic circulation, and the above reported effects of AG and UAG on glucose and insulin levels in vivo are based on measurements of systemic blood samples. Gauna et al. hypothesized that, concerning insulin secretion, assessment of insulin concentration in the portal vein might be more informative than that in the systemic circulation. They demonstred in anesthetized rats that UAG acted as a secretagogue of insulin in the portal vein. Moreover, this UAG-induced increase in insulin levels was abolished by the coadministration of AG. This study showed that UAG potently and dose-dependently enhances the insulin response to an intravenous glucose load in vivo [83]. This insulin secretagogue effect of UAG was marked in the portal vein, whereas it was scarcely detectable in the systemic circulation, suggesting that UAG plays an important role in glucose metabolism in the liver. Gauna et al. estimated that UAG slightly increased the fraction of insulin cleared by the liver, thus contributing to the augmentation of the portal-peripheral gradient of insulin [83]. Furthermore several studies support the possibility that ghrelin has a direct peripheral action on liver [73, 96]. Recently ghrelin levels have been found decreased in liver failure patients [101], a clinical condition with altered nutrition and glucose homeostasis. When Gauna et al. studied the effects of AG and UAG on primary hepatocytes; they confirmed that ghrelin in vitro induces a rapid increase of glucose output by primary hepatocytes, which suggests that AG modulates glucose homeostasis at least by acting directly on the liver. It was found that UAG itself exerts an inhibitory effect on glucose output and; as was seen in normal subjects in vivo, it is able to counteract the inductive effect of AG on glucose release [96]. The results obtained by different authors appear to indicate that the administration of UAG in humans might improve insulin sensitivity and secretion in subjects with relative or absolute GH deficiency and in the presence of GH.
These effects of UAG in the regulation of glucose metabolism might be of therapeutic interest for those pathological conditions characterized by insulin resistance and impaired insulin release.
3. Effects of Endogenous Ghrelin on Glucose and Insulin Levels
3.1. Studies In Vivo with GHSR Antagonists
In order to study the effects of endogenous ghrelin on glucose and insulin metabolism, many investigators used GHSR antagonists like modified GHRP-6 or YIL-781. In normal mice blockade of endogenous ghrelin by intraperitoneal injection of modified GHRP-6 markedly lowered fasting glucose concentrations in a few hours. Similarly during the intraperitoneal glucose tolerance test (IP-GTT), plasma glucose elevation was attenuated and insulin response was enhanced, showing a physiological role for endogenous ghrelin in the regulation of insulin release and blood glucose [58]. On the other hand YIL-781 did not affect fasting blood glucose levels. But, upon IP-GTT, the compound as well as modified GHRP-6 caused a decrease in the glucose excursion relative to the vehicle-treated animals. During an insulin tolerance test (ITT), YIL-781 did not alter the effect of insulin on blood glucose levels. This result, in combination with the effect of the compound on insulin secretion, demonstrates that, at least acutely, the GHSR1a antagonist YIL-781 improves glucose tolerance by promoting insulin release rather than enhancing insulin sensitivity. To evaluate whether GHSR1a antagonists could improve glucose tolerance in a disease model, YIL-781 was tested in the insulin-resistant diet-induced obesity (DIO) rat. In this model an oral dose of YIL-781 causes a reduction in glucose excursion. [92]. The data obtained by Esler et al. provide evidence that GHSR1a antagonists had no apparent effect on insulin sensitivity but improved glucose tolerance by stimulating insulin secretion. When ob/ob obese mice, which are a known genetic model of obesity and diabetes with insulin resistance, were peripherally administered with modified GHRP-6 during several days, plasma glucose levels diminished. This reduction in glucose levels was accompanied by a moderate decrease in serum insulin levels, suggesting that GHSR antagonists ameliorated insulin resistance in the long term [75].
The data obtained with GHSR1a antagonists (summarized in Table 4) suggest that these drugs could improve glucose tolerance and ameliorate insulin resistance in the long term and hence may be promising targets for pharmacological intervention in the treatment of type 2 diabetes.
Table 4.
Effects of GHSR antagonists on glucose and insulin levels.
| Species | Treatment Administration | Dose | Feeding | Measurement blood samples | Plasma glucose levels | Plasma insulin levels | Reference |
|---|---|---|---|---|---|---|---|
| Mice ob/ob | 1 IP GHSR antagonist injection/12 hours versus 1 IP placebo injection | 200 nmol GHRP-6/mouse | Ad libitum SCD | Endpoint 6-day | Diminished | Diminished | [75] |
|
| |||||||
| Mice ddY | 1 IP GHSR antagonist injection versus 1 IP placebo injection | 10 μmol GHRP-6/kg | Fasting overnight | Time course 2 hours | Diminished | Enhanced | |
| 1 IP GHSR antagonist injection versus 1 IP placebo injection | 1 μmol SPA/kg | Fasting overnight | Time course 2 hours | Diminished | Enhanced | [58] | |
| 1 IP GHSR antagonist + ghrelin injection versus 1 IP ghrelin injection | 1 μmol GHRP-6/kg +10 nmol ghrelin/kg | Fasting overnight | End point 0.5 hours | Diminished | |||
|
| |||||||
| Normal rats | 1 IP GHSR antagonist injection versus 1 IP placebo injection | 10 μmol GHRP-6/kg | Fasting overnight | End point 0.5 hours | Enhanced | [91] | |
| Gastrectomized rats | 1 IP GHSR antagonist injection versus 1 IP placebo injection | 10 μmol GHRP-6/kg | Fasting overnight | End point 0.5 hours | Enhanced | ||
|
| |||||||
| Normal rats | Oral GHSR antagonist + IP-GTT versus IP-GTT | 10 mg YIL-781/kg +2 g glucose/kg | Fasting overnight | Time course 6 hours | Diminished | Enhanced | [92] |
| Oral GHSR antagonist versus placebo | 30 mg YIL-781/kg | Fasting overnight | Time course 6 hours | Diminished | |||
| DIO rats | Oral GHSR antagonist +IP-GTT versus placebo + IP-GTT | 3 mg YIL-781/kg +2 g glucose/kg | Fasting overnight | Time course 6.5 hours | Diminished | ||
3.2. Glucose and Insulin Levels in GHSR-, Ghrelin-, and Double-Knockout Animals
The knockout (KO) animals represent a good opportunity to study endogenous ghrelin functions. Plasma ghrelin concentration is inversely correlated with body weight and body fat [102]. Moreover, considering that one of the main characteristics of exogenous ghrelin is to increase food intake, body weight, and % body fat [10, 87] it was expectable that the null animal for ghrelin and/or GHSR had marked differences in the ingestion and/or body composition; however the results obtained did not show that. It seems that the type of diet, its duration, age, and nutritional status of the animals are key factors to understand the function of the hormone in the energetic metabolism as well as its effect in the homeostasis of glucose and insulin. The results obtained in rats in relation to the metabolism of glucose and insulin in knockout animals are shown in Table 5.
Table 5.
Glucose and insulin levels in GHSR-, ghrelin-, and double-knockout animals.
| Null mice | Treatment | Dose | Food before/during experiment | Measurement blood samples | Plasma glucose levels | Plasma insulin levels | Reference |
|---|---|---|---|---|---|---|---|
| Ghrelin | KO versus wildtype | SCD 4–20 weeks old | Endpoint | Not change | Not change | [35] | |
|
| |||||||
| Ghrelin | KO versus wildtype | SCD 4–10 weeks of age | Endpoint | Not change | Not change | [103] | |
|
| |||||||
| Ghrelin | IP-GTT, KO versus wildtype | 2 g glucose/kg | SCD, fasted | Time course-2 hours | Diminished | Enhanced | [91] |
| KO versus wildtype | SCD, fed | Endpoint | Endpoint | Not change | |||
| KO HDF versus KO SCD | HFD 8–12 weeks old | Endpoint | Not change | Enhanced | |||
| IP-GTT, KO HFD versus KO SCD | 2 g glucose/kg | HFD 8–12 weeks old | Time course-2 hours | Not change | Enhanced | ||
|
| |||||||
| Ghrelin | IP-GTT, KO versus wildtype | 2.5 g glucose/kg | SCD 8-week old | Time course-2 hours | Diminished | Enhanced | [36] |
|
| |||||||
| Ghrelin | KO versus wildtype | SCD, 6 hours fast | Endpoint | Not change | Not change | [37] | |
|
| |||||||
| AG versus saline | 2.5 g glucose/kg + 1 IP injection of 150 nmol AG/kg | SCD 8-week old, 18 h fast | Time course-2 hours | Enhanced | Diminished | [36] | |
| ITT, KO versus wildtype | 0.75 U/kg | SCD 8-week old, 8h fast | Time course-2.5 hours | Diminished | |||
| Ghrelin | Hyperinsulinemic-euglycemic clamp, KO versus wildtype | SCD 8-week old | GIR enhanced during clamp | ||||
| KO versus wildtype | SCD 12-week old | Endpoint | Not change | Not change | |||
| KO.ob/ob versus wildtype.ob/ob | SCD 12-week old | Endpoint | Diminished | Enhanced | |||
| KO.ob/ob versus wildtype.ob/ob | SCD 12-week old, 24 hours fast | Endpoint | Diminished | Not change | |||
|
| |||||||
| Ghrelin | IP-GTT, KO versus wildtype | 2 g glucose/kg | SCD, 6 hours fast | Time course-2 hours | Not change | Not change | [37] |
| ITT, KO versus wildtype | 1 U/kg | SCD, 6 hours fast | Time course-2 hours | Not change | Not change | ||
|
| |||||||
| Ghrelin | O-GTT, KO DIO versus wildtype DIO | 1 g glucose/kg | HFD 8–23 weeks old, 16 hours fast | Time course-2 hours | Not change | Diminished | [33] |
|
| |||||||
| Ghrelin | KO versus wildtype | 10-week SCD + 40 days on 50% caloric restriction with SCD | Time course every 2 days | 2–16 day diminished | [34] | ||
|
| |||||||
| KO DIO versus wildtype DIO | HFD 8–23 weeks old | Endpoint | diminished | Diminished | |||
| IPGTT, KO DIO versus wildtype DIO | 1 g glucose/kg | HFD 8–23 weeks old, 16 hours fasted | Time course-2 hours | Not change | Diminished | [33] | |
|
| |||||||
| Ghrelin | Hyperinsulinemic-euglycemic clamp, KO DIO versus wildtype DIO | 10 mU insulin/kg + constant infused insulin 5 mU/kg/min + infused 20% glucose | HFD 8–23 weeks old, 16 hours fast | Time course-1.5 hours | GIR enhanced | ||
| Hyperglycemic clamp KO DIO versus wildtype DIO | 20% glucose at rates that stabilized blood glucose at 300 mg/dl | HFD 8–23 weeks old, 16 hours fasted | Time course-1.5 hours | Diminished | |||
| KO versus wildtype | 24-week SCD | Endpoint | Not change | Not change | |||
| KO versus wildtype | 24-week SCD/18 h-fasting | Endpoint | Diminished | Diminished | |||
| GHSR | KO versus wildtype | 10-week SCD + 40 days on 50% caloric restriction with SCD | Time course every two days | 2–28 day diminished | [34] | ||
| KO versus wildtype | 14-week SCD +10-week HF + 18 h-fasting | Endpoint | Not change | Not change | |||
| KO versus wildtype | 14-week SCD +10-week HF | Endpoint | Not change | Not change | |||
|
| |||||||
| IP-GTT KO versus wildtype | 2 g glucose/kg | SCD, 6 hours-fasting | Time course 2 hours | Not change | Not change | [37] | |
| GHSR | ITT KO versus wildtype | 1 U/kg | SCD, 6 hours-fasting | Time course 2 hours | Not change | Not change | |
| KO versus wildtype | SCD, 6 hours-fasting | Endpoint | Not change | Not change | |||
|
| |||||||
| GHSR | KO versus wildtype | SCD 4–19 weeks old | Endpoint | Diminished | Diminished | [104] | |
|
| |||||||
| GHSR | KO versus wildtype | SCD 8-week old | Endpoint | Diminished | Diminished | [105] | |
|
| |||||||
| Ghrelin + GHSR | IP-GTT dKO versus wildtype | 2 g glucose/kg | SCD, 6 hours-fasting | Time course-2 hours | Not change | Not change | [37] |
| dKO versus wildtype | SCD, 6 hours-fasting | Endpoint | Not change | Not change | |||
| ITT dKO versus wildtype | 1 U/kg | SCD, 6 hours-fasting | Time course-2 hours | Not change | Not change | ||
3.2.1. GHSR Knockout
Some investigators reported that GHSR knockout animals, in comparison with wild-type controls, had only a modest decrease in body weight when maintained on standard chow and similar levels of insulin in both fed and fasted states [106]. However GHSR null mice to 50% caloric restriction (CR) or fasting conditions on standard diet had lower blood glucose and insulin levels than standard diet fed wild-type (WT) mice suggesting enhanced insulin sensitivity [34]. These results were supported by other authors. Zigman et al. also observed that GHSR-null male mice showed lower blood glucose levels when maintained on a standard chow diet (SCD), and corresponding insulin levels were lower, although not always reached statistical significance [104].
It was observed that GHSR null mice had mean body weight and body composition comparable to those of their same-sex wildtype littermates when measured 1 week after weaning or exposure to standard chow. However, several weeks of exposure to HFD after weaning resulted in significantly less accumulation of both body weight and body fat content in GHSR null mice, as compared with littermate controls, and these animals presented resistance to diet-induced obesity [33, 104]. Interestingly, these differences are masked in HFD fed mice only in their adult stage; in this situation the deletion of GHSR does not prevent DIO or weight gain after weight loss [34].
Once more, in GHSR null mice fed with HFD, several measures of greater insulin sensitivity were observed, including lower fasted blood glucose and plasma insulin, lower insulin levels during glucose tolerance tests, and improved performance in hyperinsulinemic-euglycemic and hyperglycemic clamp studies [33].
On the other hand, the results obtained in RQ for GHSR null mice are discrepant. The knockout created by Nakano et al. presented decreased RQ during long-term HFD study that represents a shift in metabolic fuel preference toward the utilization of fat as an energy substrate [104]. On the contrary, Longo's animals have higher RQ, indicating a preference for carbohydrate as fuel regardless of gender or diet. These data could suggest that ghrelin's effects on metabolic fuel preference are transient and may not have a significant effect throughout the lifespan. Perhaps adult GHSR null mice are subject to metabolic adaptations especially in regard to energy intake and expenditure. However the range of RQ values was wider in knockout mice, indicating greater metabolic flexibility in these animals [33].
3.2.2. Ghrelin Knockout
When ghrelin KO animals and WT controls were exposed to prolonged and earlier HFD (after weaning), ghrelin KO mice showed mean body weight and mean body fat percentage that were lower than those of similarly treated wild-type controls [107]. This diet produced glucose intolerance and insulin resistance in wild type mice [91, 107]. By contrast, ghrelin knockout mice fed with HFD showed close to normal glucose responses and markedly enhanced insulin responses to IP-GTTs compared with control ghrelin knockout mice fed with SCD [91]. As a possible underlying mechanism Dezaki et al. suggested that lack of ghrelin and its insulinostatic activity may raise the maximal capacity of glucose-induced insulin release and enable islets to secrete more insulin to meet an increased demand associated with HFD–induced obesity, thereby achieving normoglycemia [91]. Moreover Ghr KO mice on the HFD presented lower levels of glucose and insulin as well as lipids compared with wild-type on this diet; hence ghrelin as well as GHSR null mice exposed to HFD after weaning exhibit greater glucose tolerance. The results of GTTs and ITTs were similar to those previously observed for the same authors with pharmacological blockade of ghrelin action [58], reinforcing the concept that endogenous ghrelin serves as a regulator of insulin release and of glycemia. However, when ghrelin null mice and wild type mice were subjected to acute exposure to HFD late in life, slight differences in body composition between ghrelin KO animals and wild-type controls were reported, and no change in glucose and insulin levels [103]. Comparable results were obtained in animals fed with standard chow, where insulin and glucose levels did not display changes [35]. Moreover these animals did not display differences in cumulative food intake on standard chow or body weight change and food intake in response to reexposure to food following a fast [35]. However, Sun et al. realized several studies where they observed, that compared to WT, ghrelin KO mice exhibited significantly lower glucose levels after IP-GTT and correspondingly higher levels of insulin. In addition, the initial insulin response at 15 minutes was significantly higher in the ghrelin KO compared to WT mice [36]. When ghrelin KO mice were subjected to 50% caloric restriction, they had lower blood glucose levels than their WT littermates suggesting that ghrelin would be involved in providing a counterregulatory glucose response during negative energy balance [34].
In another line of ghrelin knockout mice, glucose levels were monitored in lean mice (wild-type and ghrelin KO) and obese mice (wild type ob/ob and ghrelin KO ob/ob) at different ages [36]. The lean mice were euglycemic; as expected, glucose and insulin levels were elevated both in ob/ob and ghrelin KO ob/ob mice. However blood glucose was elevated at age 4 weeks in ob/ob mice and at 6 weeks in ghrelin KO ob/ob mice, and although obesity was as severe as in ob/ob mice, ghrelin KO ob/ob exhibited lower glucose levels and their blood glucose normalized upon fasting. Hence, ablation of ghrelin markedly improved glucose homeostasis in ob/ob mice [36]. The improvement in glucose homeostasis in ghrelin KO ob/ob mice was accompanied by increased serum insulin levels. Remarkably, compared to ob/ob mice, ghrelin KO ob/ob mice displayed reduced blood glucose concentrations after IP-GTT, which was accompanied by increased insulin secretion [36]. When ghrelin KO mice, maintained from weaning on regular chow, were subjected to IP-GTT, ghrelin treatment produced higher blood glucose and markedly lowers insulin levels, showing that ghrelin acutely suppresses insulin release, suggesting that the improved glucose tolerance which was observed in ghrelin KO ob/ob mice fed with HFD during IP-GTT could be a consequence of ghrelin-ablation. Moreover, ghrelin ablated mice presented greater reductions in glucose levels 30 minutes following ITT suggesting increased insulin sensitivity. When the authors subjected WT and ghrelin KO mice to euglycemic hyperinsulinemic clamp studies, basal hepatic glucose production rate was the same in both genotypes. But during the low-dose insulin clamp, suppression of glucose production was higher in ghrelin KO mice, proposing once more that the liver of ghrelin KO mice was more sensitive to insulin. Furthermore, an increase in glucose infusion rate (GIR) and an increase in GDR were detected, indicating that besides increasing glucose-induced insulin secretion, ghrelin ablation increased peripheral insulin sensitivity and improves glucose tolerance [36].
Wortley et al. found a trend toward decreased weight and leaner body composition in male ghrelin knockout mice after 6 weeks on the HFD, which could be explained by a decrease in RQ observed only in these animals; therefore the constitutive absence of ghrelin causes a distinct shift toward lipid metabolism during consumption of an HFD [103].
3.2.3. Ghrelin/Ghrelin Receptor Double Knockout (dKO) Mice
Pfluger and colleagues created ghrelin/ghrelin receptor double knockout mice. Plasma glucose and plasma insulin levels did not differ between aged WT and dKO mice after an overnight fast. An IP-GTT overall failed to reveal significant differences in glucose tolerance between genotypes. Mice deficient in either ghrelin, GHSR, or both showed lower glucose peak levels at a single time point (15 minutes after the injection) suggesting a slightly faster release of insulin. Mice were subjected to an ITT; in ghrelin KO mice glucose levels were similar to WT mice. In dKO and GHSR KO mice, glucose levels, however, dropped more rapidly. In general, glucose levels of dKO and GHSR mice tended to remain lower throughout the 120 minutes of the ITT, compared with WT mice. However, although integrated glucose levels in both GHSR KO and dKO mice tended to be lower compared with WT control mice, the deficiency of ghrelin, its receptor, or both did not seem to have a major impact on overall insulin sensitivity or the overall regulation of glucose homeostasis. They observed substantial but mostly insignificant trends in glucose tolerance and insulin sensitivity. Importantly, all these data were obtained from mice maintained on normal standard chow diet [37].
Pfluger et al. speculated that their mouse mutants still may exhibit some level of ghrelin signaling, although by definition they genetically deleted ghrelin [9], its putative ghrelin associated peptide [108], ghrelin splice variants [109], and the constitutively active ghrelin receptor GHSR [110]. For this reason the authors suggested that the existence of both additional ligand and additional receptor, coded for by genes other than the ghrelin and the GHSR gene, could explain why the dKO mouse shows a phenotype that still has to be categorized as very mild.
In summary, the results obtained with knockout animals seem to indicate that ghrelin is not a critical orexigenic factor. Nevertheless, the ghrelin/GHSR pathway plays a role in glucose homeostasis by regulating insulin sensitivity and glucose sensing. If it was confirmed that ghrelin ablation restores the first-phase of insulin secretion, as observed in ghrelin knockout ob/ob mice, [36] this could have clinical relevance, because in humans the loss of first-phase insulin secretion is predictive for the development of type 2 diabetes [111]; therefore, in subjects at risk for type 2 diabetes, treatment with a ghrelin antagonist may prove beneficial. Kelley and colleagues proposed a central pathophysiological construct to describe the altered metabolism associated with insulin-resistant and glucose-intolerant states: the concept of “metabolic inflexibility” [112]. Metabolically normal people can adapt to the discontinuities in fuel availability and utilization present in daily life, whereas diabetic people cannot. Metabolic inflexibility means that insulin-resistant individuals are unable to efficiently increase carbohydrate utilization, even when carbohydrates are plentiful. This is a rewording of the essence of impaired glucose tolerance (and insulin resistance). The results obtained in RQ of GHSR KO mice seem to indicate greater metabolic flexibility and hence improve glucose tolerance. When null mice were fed with either SCD or HFD, their body weights were not different from that of their WT littermates on the same diet. However ghrelin and GHSR null mice were resistant to DIO when were fed with HFD immediately after weaning. But ablation of the ghrelin/GHSR signal does not prevent DIO raised on SCD and then fed with HFD as adults. Considering these data it would be possible to conclude that the loss of ghrelin signalling protects against several fatty diet-induced features of metabolic syndrome and improves insulin sensitivity. But all these results should be taken with caution, considering that the age of exposition and the type of diet seem to be key factors to observe the effect of ghrelin on glucose and insulin metabolism.
3.3. Glucose and Insulin Levels in GHSR and Ghrelin Transgenic Animals
There are some studies realized with ghrelin transgenic mouse with overexpression of ghrelin in different tissues or cellular types (Table 6). Many of them presented plasma UAG levels higher than those of their nontransgenic littermates whereas that plasma acylated ghrelin levels did not change [27, 38, 39]. Hence these models can serve to study the role of desacyl as well as acylated ghrelin in the regulation of glucose metabolism and insulin release.
Table 6.
Relation between overexpression of ghrelin in different tissues or cellular types and glucose-insulin levels.
| Transgenic animals | Ghrelin levelstransgenic versus wildtype | Treatment | Food before/during experiment | Treatment duration | Plasma glucose levels transgenic versus wildtype | Plasma insulin levels transgenic versus wildtype | Reference |
|---|---|---|---|---|---|---|---|
| Ghrelin is overexpressed inadipose tissue | AG: not change UAG: enhanced | Nothing | Ad libitum | Endpoint | Enhanced | [26] | |
| IP-GTT | 16-h fast | 2.5 hours | Diminished | ||||
| IP-ITT | 16-h fast | 2.5 hours | Diminished | ||||
|
| |||||||
| Ghrelin is overexpressed in stomach and hypothalamus | AG: enhanced UAG: enhanced | IP-GTT | 18-h fast | 2.5 hours | Enhanced | Diminished | [113] |
| IP-ITT | 4-h fast | 2.5 hours | Not change | Not change | |||
|
| |||||||
| Ghrelin is overexpressed in pancreas | AG: not change UAG: enhanced | Nothing | Overnight fast | Endpoint | Not change | Not change | [39] |
| IP-GTT | Overnight fast | 2 hours | Not change | Diminished | |||
| IP-GTT | Overnight fast | 2 hours | Not change | Diminished | |||
| ITT | 4-h fast | 3 hours | Not change | Not change | |||
|
| |||||||
| Ghrelin is overexpressed in hypothalamus, cortex and liver | AG: enhanced UAG: enhanced | IP-GTT | 20-h fast | 2 hours | Enhanced | Not change | [114] |
| Ghrelin is overexpressed in hypothalamus, cortex and liver | AG: not change UAG: enhanced | IP-GTT | 20-h fast | 2 hours | Not change | Not change | |
|
| |||||||
| Ghrelin is overexpressed in wide variety of tissues | AG: not change UAG: enhanced | Nothing | Ad libitum | Endpoint | Not change | Not change | [27] |
Iwakura and colleagues developed and analyzed rat insulin II promoter-ghrelin transgenic mice (RIP-G Tg) in which pancreatic ghrelin concentration was higher than that of nontransgenic littermates; moreover in control mice ghrelin was not detected in β-cells by immunohistochemistry. Ghrelin transgene driven by RIP was considered to be expressed in β-cells, although higher expression levels of ghrelin mRNA were also found in the brain of RIP-G Tg compared with that of nontransgenic littermates. When these animals were subjected to IP-GTT, plasma insulin levels were significantly lower in Tg mice than those in nontransgenic littermates, although there was no significant difference in plasma insulin levels between RIP-G Tg and nontransgenic littermates on the fasting state [39]. The glucose-stimulated insulin secretion of RIP-G Tg was decreased without changes in glucose levels, but there were no abnormalities with the arginine-induced insulin secretion, pancreatic histology, pancreatic insulin mRNA levels, and insulin content in the RIP-G Tg. When the authors did several tests from isolated islets of RIP-G Tg, they found that insulin secretion as well as immunoreactivity of glucose transporter in the pancreatic β cell, in RIP-G Tg β cells, was indistinguishable from that of nontransgenic littermates, indicating that insulin secretion was not affected by overexpression of ghrelin transgene in vitro, although it was affected in vivo [39]. When these animals were subjected to ITT, they showed a tendency to lower blood glucose levels. Considering the results, the authors suggested that the suppression of insulin secretion of RIP-G Tg is likely due to the effect of desacyl ghrelin on insulin sensitivity [39]. Nevertheless these results do not agree with others studies.
Reed et al. created mice with ghrelin overexpressed in neurons using the neuron-specific enolase (NSE) promoter sequences and mouse ghrelin cDNA (NSE-ghrelin). Ghrelin expression in NSE-ghrelin brain tissues was increased compared with wild-type mice; it was also increased to a much smaller extent in liver of these mice, but in stomach or duodenum did not differ from wild-type mice. They worked with two lines of NSE-ghrelin mice: one line with increased circulating AG and UAG (L43) and one line with only UAG (L73). In both lines young NSE-ghrelin mice had normal glucose tolerance; however, L43 NSE-ghrelin mice, but not L73 mice, developed glucose intolerance at 32 week of age. Despite the impaired glucose tolerance in L43 mice, insulin levels did not differ from those of wild-type mice [114]. However, unlike the studies from Iwakura et al. plasma insulin levels did not change after IP-GTT in those animals with high levels of UAG (L73). The differences between both studies can be the consequence of several factors like age or others. In another line of transgenic mice, Zhang et al. created animals in which the ghrelin gene is overexpressed in adipose tissue via the fatty acid-binding protein-4 (FABP4) promoter. Transgenic mice overexpressing the ghrelin gene in adipose tissue demonstrated significant increases in plasma concentrations of UAG, whereas ghrelin remained unchanged. Overexpression of ghrelin from the FABP4 promoter reduced the weight of white adipose tissues and resistance to HFD-induced obesity [26]. Alterations in glucose tolerance and insulin sensitivity tests were detected in FABP4-ghrelin transgenic mice. When these animals were subjected to IP-GTT, glucose levels were significantly lower than in controls; however FABP4-ghrelin transgenic mice had a greater hypoglycemic response to insulin administration than control animals. It seems that UAG improves glucose tolerance and insulin sensitivity, providing more evidences that UAGs play a role in the regulation of glucose metabolism. These data are strengthened by the observation that plasma insulin levels are elevated in transgenic mice [26].
Recently, Bewick et al. generated a mouse model with increased ghrelin expression and production in stomach and brain. Ghrelin transgenic mice exhibited increased circulating AG and total ghrelin which was associated with hyperphagia and increased energy expenditure. These animals were subjected to IP-GTT and ITT; the animals were glucose intolerant due to an inhibition of glucose-stimulated insulin release but without change in insulin sensitivity [113].
4. Mechanism of Action
In order to understand how ghrelin can modify glucose and insulin homeostasis, it is important to study the mechanism of action exerted by ghrelin in tissues implied in carbohydrate metabolism.
4.1. Liver
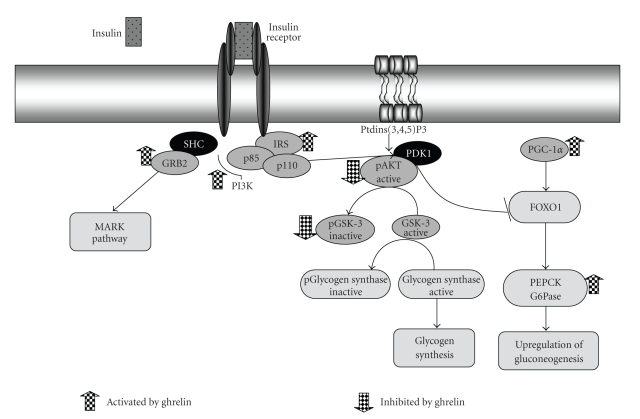
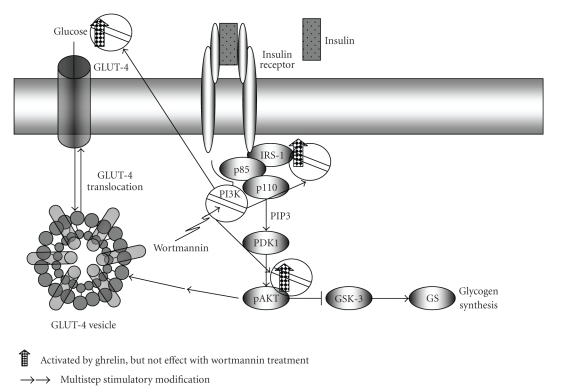
De novo synthesis of glucose in the liver from precursors such as lactate, gluconeogenic amino acids, and glycerol is a central mechanism to provide the organism with glucose in times of starvation [115], a natural situation in which ghrelin levels are increased [10, 30]. On the other hand, when glucose is directly available from external resources, gluconeogenesis is dispensable and consequently needs to be shut off. Integration of these events is complex and occurs through various hormonal and nutritional factors. The principal parameters affecting hepatic glucose output are the concentrations of the available glucogenic substrates and the activity of a few regulatory enzymes. The activity of the key gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and G6Pase is regulated by transcriptional and nontranscriptional mechanisms, whereas the third key enzyme fructose-1,6-bisphosphatase (FBPase) is also regulated through competitive inhibition by fructose 2,6-bisphosphate. Insulin is the most important hormone that inhibits gluconeogenesis, through the activation of the insulin receptor (IR). It acts predominantly by suppressing the expression of the genes for the key gluconeogenic enzymes PEPCK and G6Pase [116]. In the H4-II-E-cells (rat hepatoma cell line) and HepG2 cells (human hepatocellular carcinoma cell line) ghrelin was shown to stimulate insulin receptor substrate 1º(IRS1) and its downstream molecules, including growth factor receptor-bound protein 2 (Grb2) and mitogen-activated protein kinase (MAPK). Whereas on the other hand, it diminished phospho protein kinase B (pAKT) and phospho-glycogen synthase kinase (pGSK) levels in both cell lines and upregulated gluconeogenesis in H4-II-E-cells by attenuating the effect of insulin on the expression on PEPCK [73].
AKT is a key protein kinase downstream of the insulin receptor [117] and its activation plays a key role in suppressing hepatic gluconeogenesis [118, 119], since GSK-3, which phosphorylate glycogen synthetase (GS) is inhibiting, is phosphorylated by AKT, and this phosphorilation inactivates GSK-3 kinase activity, suppressing hepatic gluconeogenesis resulting in enhanced glycogen deposition [118].
Forkhead box O1 (FOXO1) and peroxisome proliferator activated receptor-γ-coactivator (PGC)-1α are two transcriptional components that are targets of insulin signalling and that can activate the process of gluconeogenesis in liver. FOXO1 has been shown to bind directly to the promoters of gluconeogenic genes and activate the process of glucose production [120–122]. It is directly phosphorylated by AKT. This phosphorylation results in exclusion of FOXO1 from the nucleus. A second transcriptional component controlled by insulin and having a role in gluconeogenesis is the coactivator PGC-1α. It is induced in the liver during fasting and is elevated in several models of diabetes or deficiency in insulin signalling. Notably, expression of PGC-1α at physiological levels turns on the entire program of gluconeogenesis [123]. PGC-1α hepatic transcription has been reported to be downregulated by AKT activation through forkhead transcription factor FOXO1 phosphorylation and nuclear exclusion [124].
Barazzoni et al. observed that in rats sustained ghrelin administration reduced hepatic phospho/total-AKT (P/T-AKT) and P/T-GSK [86]. These changes in AKT-GSK signalling were associated with enhanced PGC-1α expression. Reduced liver AKT signaling could potentially contribute to concomitant blood glucose increments, preserving hepatic glucose production in calorie-restricted status [86].
The routes that have been modified after treatments with ghrelin in liver and which could modify plasma glucose levels are represented in Figure 1.
Figure 1.
Regulation of hepatic gluconeogenesis and glycogen synthesis by ghrelin. Insulin activates the insulin receptor tyrosine kinase (IR), which phosphorylates and recruits different substrate adaptors. AKT is a key protein kinase downstream of the insulin receptor and its activation plays a key role in suppressing hepatic gluconeogenesis, since GSK-3, which phosphorylate glycogen synthetase (GS) is inhibiting, is phosphorylated by AKT suppressing hepatic gluconeogenesis, resulting in enhanced glycogen deposition. Sustained ghrelin administration in rats reduced hepatic AKT-GSK activation and enhanced PGC-1a expression, suggesting upregulation of gluconeogenesis and downregulation of glyconeogenesis.
4.2. Pancreas
Insulin secretion is accurately linked to blood glucose levels in the physiological range. The role of the β-cells is to sense an increase in the concentration of nutrients in the blood and to synthesize, package, and release insulin to control blood glucose homeostasis. Various agents as amino acids (particularly arginine and leucine) and fatty acids can increase the secretion of insulin, but only in the presence of facilitating concentrations of glucose (above 3 mM), whilst nonmetabolizable analogues of glucose such as galactose or fructose are inactive as secretagogues [125]. The above fuel secretagogues are initiators of secretion, but there are also other agents including neurotransmitters, glucagon-like peptide (GLP-1), gastric inhibitory peptide (GIP), and pituitary adenylate cyclase-activating polypeptide (PACAP) that act as “potentiators”, enhancing secretion only at permissive concentrations of fuel secretagogues. These molecules usually act via G-protein coupled receptors and the generation of classical second messengers such as cAMP and Ca2+ [126]. The first studies about stimulus-secretion coupling in β-cells early concluded that glucose must be metabolized by β-cells to induce insulin secretion, Ca2+ has an essential role in insulin secretion, and pancreatic β-cells are electrically excitable [125].
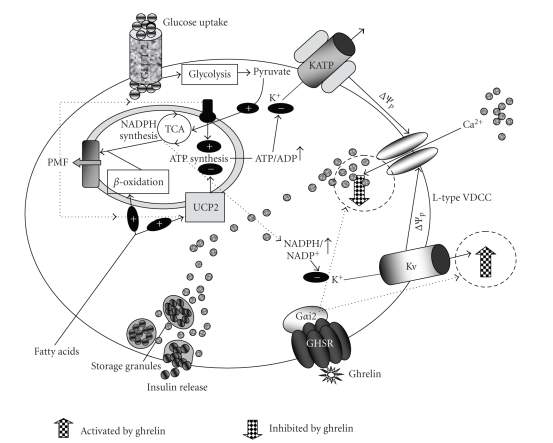
Islet β-cells are equipped with high-capacity glucose transporters located at the plasma membrane that are known as glucose transporters-2 (GLUT-2) [127]. GLUT-2 is required for efficient glucose-stimulated insulin secretion, as demonstrated by studies in transgenic mice [128, 129]. β-cells contain a high Km glucokinase (glucose-phosphorylating hexokinase, GK), which displays strongly cooperative kinetics and has thus been termed the β-cell “glucose sensor.” The reduction in β-cell GK levels was associated with reduced capacity to secrete insulin in response to glucose [130]. Glucose stimulation of insulin secretion involves two pathways: the triggering of ATP-sensitive K+channel- (KATP-) dependent pathway, and the amplifying of KATP channels-independent pathway. The rise in blood glucose induces an increase in β-cell glucose metabolism, resulting in increased production of ATP from several sources: glycolysis, mitochondrial glucose oxidation, and active shuttling of reducing equivalents from the cytosol to the mitochondrial electron transport chain. The resultant increase in ATP/ADP ratio inhibits KATP channels, depolarizing the plasma membrane, leading to opening of the voltage-dependent calcium channels (VDCCs), which allows calcium influx. The resultant intracellular calcium concentration ([Ca2+]i) rise triggers exocytosis of the insulin-containing granules (reviewed in [131]). However, an expanding bulk of data also makes it apparent that this KATP-channel dependent mechanism of glucose-stimulated insulin secretion does not fully describe the islet glucose response, and signals other than changes in ATP: ADP ratio have been increasingly implicated as important regulators of insulin secretion in recent years. The voltage-dependent K+ channels (Kv) [132] are thought to repolarize glucose-stimulated action potentials and inhibit Ca2+ entry through voltage-gated Ca2+ channels; therefore, Kv channels serve as negative regulators of insulin secretion, and Kv channel antagonists are insulinotropic in a glucosedependent manner. Kv channels are comprised of the pore-forming α subunits (Kv2.1 is thought to be the predominant isoform in islet β-cells) and regulatory β-subunits, analogous to the pore-forming and regulatory subunits of the KATP channel complex. Kv channel β–subunits are proposed to act as intracellular redox sensors, and an increase in cytosolic NADPH : NADP ratio in patch-clamped-cells was shown to be associated with an increased rate of inactivation of the Kv channel [133]. Inhibition of Kv channels by NADPH, derived from pyruvate cycling, could serve as a logical complementary mechanism to ATP regulation of KATP channel activity, since suppression of Kv channels would slow membrane repolarization, allowing the effects of KATP channel inhibition to be sustained through a second phase of insulin secretion (Figure 2). But this model is not fully established (reviewed in [134]).
Figure 2.
β-cell mechanisms of insulin release and its regulation by ghrelin. When the plasma glucose concentration rises, β-cells oxidize it. Glucose oxidation establishes a protonmotive force (PMF) that drives ATP synthesis, increasing the ATP/ADP ratio. This causes closure of KATP-channels, depolarisation of the plasma membrane potential (Δψp) and Ca2+ flux into the cell, triggering insulin release. UCP2 activity dissipates the protonmotive force, lowering ATP/ADP. Ghrelin directly acts on the β-cell and via PTX–sensitive mechanisms attenuates glucose-induced [Ca2+]i signalling partly through enhancement of TEA-sensitive delayed outward K+ currents resulting in decrease plasma insulin levels. PTX catalyzes the ADP-ribosylation of the α subunits of the heterotrimeric G proteins Gi, Go, and Gt. This prevents the G proteins from interacting with G protein-coupled receptors on the cell membrane thus interfering with intracellular communication. Since the Gα subunits remain in their GDP-bound, inactive state, they are unable to inhibit adenylyl cyclase, thus keeping levels of adenylyl cyclase and cAMP elevated. PTX inhibited a number of insulin-stimulated cellular events, such as glucose transport and its metabolism. Antisense oligonucleotide specific for Gαi2-subunit of G proteins blocks the effects of ghrelin on [Ca2+]i and insulin release. Hence ghrelin presumably suppresses glucose-induced insulin release via Gαi2- and Kv channel–mediated attenuation of Ca2+ signalling in β-cells.
In rat isolated islets, several works showed that endogenous and exogenous ghrelin suppressed glucose-induced insulin release [58, 91, 92, 135]. Dezaki et al. presented ghrelin signalling in β-cells. They observed that in rats ghrelin of both endogenous and exogenous origin resulted in pertussis toxin- (PTX-) sensitive decrease in plasma insulin concentrations, contrasting with PTX-insensitive increase in GH levels by ghrelin [58, 136]. PTX catalyzes the ADP-ribosylation of the α subunits of the heterotrimeric G proteins Gi, Go, and Gt. This prevents the G proteins from interacting with G protein-coupled receptors on the cell membrane, thus interfering with intracellular communication. Since the Gα subunits remain in their GDP-bound, inactive state, they are unable to inhibit adenylyl cyclase, thus keeping levels of adenylyl cyclase and cAMP elevated [137]. In intact cells, PTX inhibited a number of insulin-stimulated cellular events, such as glucose transport and its metabolism. The function of endogenous ghrelin was assessed by the effects of GHSR antagonist in vivo and in rats treated with ghrelin and PTX. In addition, studies with isolated islets from ghrelin-KO mice observed that modified GHRP-6 increased plasma insulin concentrations after IP administration, indicating suppression of insulin levels by endogenous ghrelin. The insulinostatic effect of ghrelin was unaltered by pretreatment with phospholipase C (PLC) inhibitor. However the effects of endogenous and exogenous ghrelin on insulin levels were not observed in PTX-treated rats. In islets isolated from ghrelin-KO mice, glucose-induced insulin release was greater than those from wild-type mice. This enhancement was blunted by pretreatment with PTX. They observed that ghrelin increased Kv currents and that tetraethylammonium (TEA), a Kv channel blocker, eliminated the ability of ghrelin to suppress insulin release. Furthermore, ghrelin treatment-inhibited glucose induced [Ca2+]i increases in β-cells. All the effects of endogenous and exogenous ghrelin on Kv and [Ca2+]i as well as insulin release were blunted in the presence of PTX. This finding suggests that glucose-induced insulin release in islets is markedly decreased by endogenous ghrelin. Endogenous ghrelin in islets restrict glucose-induced insulin release via the following mechanism: ghrelin directly acts on the β-cell GH secretagogue receptor and via PTX–sensitive mechanisms attenuates glucose-induced [Ca2+]i signalling, partly through enhancement of TEA-sensitive delayed outward K+currents [58, 136]. When the islet β-cells were treated with the antisense oligonucleotide specific for Gα i2-subunit of G proteins, the effects of ghrelin on [Ca2+]i and insulin release were abolished (Figure 2). These findings demonstrate that ghrelin suppresses glucose-induced insulin release via Gα i2- and Kv channel–mediated attenuation of Ca2+ signalling in β-cells [136].
All these data reveal that endogenous ghrelin in islets acts on β-cells to restrict glucose-induced insulin release, at least partly via attenuation of Ca2+ signaling, and that this insulinostatic action may be implicated in the upward control of blood glucose. These unique signaling mechanisms and molecules mediating the insulinostatic action of ghrelin on β-cells provide potential therapeutic targets for the prevention and treatment of type 2 diabetes and hyperinsulinemia [58, 136].
4.3. Adipocytes
The insulin stimulation of glucose uptake in adipose and muscle tissue occurs through a complex and as yet incompletely defined signalling pathway acting through the insulin receptor tyrosine kinase. The primary effect is to promote the movement of the GLUT-4 protein from intracellular storage sites to the plasma membrane. In the basal state, GLUT-4 is localized to a morphologically defined “tubulovesicular system” present in the intracellular compartment, while in the presence of insulin, GLUT-4 is immunolocalized to the plasma membrane of fat cells [138]. The rate-limiting step at which insulin stimulates uptake of glucose in fat is the translocation of GLUT-4 to the plasma membrane [139]. At least two discrete signalling pathways have been implicated in insulin-regulated GLUT-4 translocation. The first involves the lipid kinase phosphatidylinositol 3-kinase (PI3K) [140, 141], and the second involves the proto-oncoprotein c-Cbl [142, 143]. When insulin binds to its receptor induces a conformational change in the receptor and leads to activation of its tyrosine-kinase domain. On activation, the receptor phosphorylates several proximal substrates, including members of the IRS and c-Cbl. Tyrosine-phosphorylated IRS proteins, which are thought to be held in close proximity to the plasma membrane through association with the underlying cytoskeleton, recruit more effectors molecules, such as PI3K, to this location. Two important targets of PI3K in muscle and fat cells that have been shown to have a role in insulin-stimulated GLUT-4 translocation are the AKT and the protein kinase C (PKC). PI3K activates AKT by generating polyphosphoinositides in the inner leaflet of the plasma membrane. This acts as an anchorage site for AKT through its pleckstrin homology domain, thereby bringing it in close proximity to its upstream regulatory kinase, phosphatidylinositol-dependent kinase-1 (PDK-1). The second putative signalling pathway that has been shown to have a role in insulin-stimulated GLUT-4 translocation operates independently of PI3K and involves a dimeric complex that comprises c-Cbl and the c-Cbl-associated protein CAP. Intriguingly, whereas many growth factors trigger the activation of PI3K, AKT, and PKC in many cell types, aspects of the c-Cbl–CAP pathway, including the tyrosine phosphorylation and the expression of CAP, seem to be unique to muscle and fat cells [144]. Patel et al. examined the expression of GHSR1a in discrete adipose tissue depots and while GHSR1a expression was detected in the epididymal and pericardial deposits, it was not found in the perirenal, subcutaneous, and omental deposits. Ghrelin and des-acyl ghrelin did not affect basal deoxyglucose uptake in adipocytes from the epididymal fat deposits. However, treating isolated epididymal adipocytes with ghrelin in the presence of insulin increased insulin-stimulated deoxyglucose uptake. Des-acyl ghrelin had no significant effect on insulin-stimulated deoxyglucose uptake in isolated epididymal adipocytes. Ghrelin had no effect on basal deoxyglucose uptake in isolated perirenal adipocytes, which do not express the GHSR1a mRNA. As expected, insulin increased glucose uptake, but ghrelin in the presence of insulin did not further increase this response. Furthermore, des-acyl ghrelin did not increase insulin-stimulated deoxyglucose uptake in perirenal adipocytes. These data suggest that ghrelin may act synergistically to potentiate insulin-stimulated glucose uptake and may improve insulin sensitivity [145]. Interestingly, ghrelin did not affect insulin-stimulated glucose uptake in perirenal adipocytes, which do not express GHSR1a, and des-acyl ghrelin, which does not bind to GHSR1a, did not influence insulin-stimulated glucose uptake in epididymal adipocytes The effects of ghrelin on adipocyte glucose uptake might be expected to result in fatty acid accumulation and an increase in adiposity in the long term [145]. Kim and colleagues incubated terminally differentiated 3T3-L1 adipocytes with insulin or/and ghrelin overnight and assayed glucose transport. Insulin and ghrelin increased glucose transport and the cotreatment of insulin and ghrelin induced a further increase in glucose transport. In addition, ghrelin treatment induced increases IRS-1 and AKT phosphorylation, but when the adipocytes were treated with wortmannin, a PI3K inhibitor, completely blocked this ghrelin induced increase in glucose transport and phospho-AKT expression [146], suggesting that PI3K/AKT activation may mediate the effect of ghrelin on glucose transport in these adipocytes (Figure 3).
Figure 3.
Glucose uptake in adipose tissue and its regulation by ghrelin. Insulin activates the IR, which phosphorylates and recruits different substrate adaptors such as the IRS family of proteins. Tyrosine phosphorylated IRS then displays binding sites for numerous signaling partners. Among them, PI3K has a major role in insulin function, mainly via the activation of the AKT/PKB and the PKCz cascades. Activated AKT induces glycogen synthesis, through inhibition of GSK-3. Insulin stimulates glucose uptake in muscle and adipocytes via translocation of GLUT-4 vesicles to the plasma membrane. GLUT-4 translocation involves the PI3K/AKT pathway. Ghrelin treatment induced increases IRS-1 and AKT phosphorylation, but when the adipocytes were treated with wortmannin, a PI3K inhibitor, completely blocked this ghrelin induced increase in glucose transport and phospho-AKT expression, suggesting that PI3K/AKT activation may mediate the effect of ghrelin on glucose transport in these adipocytes.
All these data suggest that the direct effects of ghrelin on insulin-stimulated glucose uptake are mediated by the GHSR1a and PI3K/AKT activation.
5. Pharmacological Uses of Ghrelin on Glucose-Insulin Homeostasis
Overt diabetes mellitus is defined clinically by fasting or postprandial hyperglycemia or an abnormally increased glucose excursion in response to a defined glucose load. Insulin resistance, measured as impaired glucose disposal in a hyperinsulinemic-euglycemic clamp study, is one of the earliest detectable disorder and is considered a cardinal pathophysiologic feature [147]. Fasting hyperinsulinemia is also present early in the disease process and is thought to be a compensatory mechanism to maintain euglycemia in the setting of insulin resistance [148]. Even while maintaining a healthy lifestyle, most patients need pharmacological intervention which might consist of one or a combination of the following oral medications: sulfonylureas, glinides, incretin mimetics, α-glucosidase inhibitors, metformin, or thiazolidinediones. However 30%–40% of patients are not adequately controlled by these therapies and require subcutaneous insulin injections intended to restore normoglycemia, but they can inadvertently lead to hypoglycemia, a potentially fatal consequence. Thus, new drugs and novel methods of treatment are needed. Among diabetic patients, 10%–20% fall into the category of insulin-dependent diabetes mellitus (IDDM) or type 1 diabetes, which generally appears before age 40, frequently in adolescence, and results from autoimmune destruction of insulin producing pancreatic β-cells. Type 1 diabetic patients depend on insulin administration for their survival. Noninsulin-dependent diabetes mellitus (NIDDM) or type 2 diabetes is far more common than IDDM, affecting 80%–90% of diabetic patients. The prevalence of obesity and type 2 diabetes continues to increase at alarming rates [149]. Type 2 diabetes is a prototypic complex, polygenic disease with a strong heritable component, which is also heavily influenced by environmental factors, especially diet and physical activity. It appears that altered communication among tissues and loss of the ability of tissues to adapt to changing metabolic states play a critical role in the altered glucose homeostasis that leads to the development of type 2 diabetes. It is characterized by a combination of factors that affect the organism's ability to respond to insulin. The condition has two hallmark features: (1) insulin resistance and (2) compromised function of the pancreatic β-cell, such that insulin secretion is insufficient to counterpart the degree of insulin resistance. There is general agreement that type 2 diabetes, unlike IDDM, is tightly associated with obesity. Over 80% of individuals with type 2 diabetes are obese. However, only 10% of obese individuals are diabetic. In the prediabetic phase, when insulin resistance has already begun, the β-cell actually hypersecretes insulin despite normal blood glucose levels. What has defied explanation is precisely what causes this insulin resistance in the first place and how it relates in a temporal sense to the accompanying hyperinsulinemia.
Ghrelin receptor modulation could be clinically useful for different situations related with glucose-insulin homeostasis (Table 7). Several works demonstrated that ghrelin concentrations are negatively associated with fasting insulin levels, the prevalence of type 2 diabetes and insulin resistance in humans, regardless of race [102, 150, 151]. The data obtained since ghrelin discovery show that both the acylated and unacylated molecules are actively involved in the acute and long-term control of glucose metabolism and insulin sensitivity in humans, which might enable new treatment modalities for the many disorders in which insulin sensitivity is disturbed. Thus, pharmacological, immunological, and genetic blockade of ghrelin or ghrelin action in pancreatic islets all markedly enhanced glucose-induced insulin release and improve the diabetic condition. Hence ghrelin inhibition could be useful for the treatment of diabetes [152, 153].
Table 7.
Potential therapeutic uses of ghrelin agonists and antagonists on glucose-insulin homeostasis.
| Ghrelin agonists | Ghrelin antagonists |
|---|---|
| Insulinoma | Type 2 Diabetes mellitus |
| Anorexia nervosa | Metabolic syndrome |
| Cachexia of malignancy | Obesity |
On the other hand the ability to efficiently build fat reserves in times of nutritional abundance appears to have resulted from evolutionary pressure to protect against subsequent periods of food scarcity. The tendency to efficiently store fat in times of caloric excess appears to have become paradoxically maladaptive in settings of continuous food availability, as indicated by the present epidemic of obesity in Western societies. The data obtained in the last years seem to indicate that ghrelin may be one of the primary mechanisms by which an individual can sense changes in nutrient availability and trigger biological responses that modulate the efficiency of energy storage (and particularly fat deposition) during periods of fuel overflow or after a period of scarcity of nutrients. At present, ghrelin is the only peripheral orexigenic factor that is effective upon its intravenous administration [81]. Put in this context, the blockade of the route of ghrelin could prove useful in controlling adiposity in human obesity, as blockers of the orexigenic signal from the gastrointestinal tract to the brain, or diminishing the ability to efficiently store fat reserves. Inverse agonists of the ghrelin receptor, by blocking the constitutive receptor activity, might lower the set-point for hunger between meals [110, 154]. All these data suggest that ghrelin-ghrelin receptor modulation has the potential to improve the diabetic condition by promoting glucose-dependent insulin secretion and promoting weight loss.
In contrast, ghrelin may be useful as an orexigenic agent for the treatment of eating disorders such as anorexia nervosa. Administration of ghrelin can stimulate appetite and improve the nutritional status of these patients. However, plasma ghrelin concentrations in anorexia nervosa are high, indicating a situation of ghrelin resistance [100]. In fact, circulating ghrelin levels have been found altered in different clinical situations, like renal failure or hepatic failure [101, 155]. Ghrelin-derived drugs could also be useful in all the clinical situations associated with cachexia, such as malignancy, advanced cardiac failure, renal failure, postoperative patients, and human immunodeficiency virus-lipodystrophy. In Table 8 we summarize putative ghrelin effects on glucose-insulin homeostasis and related physiological actions.
Table 8.
Summary of putative ghrelin effect on glucose-insulin homeostasis and related physiological actions.
| Ghrelin effects on glucose-insulin homeostasis |
|---|
| Increase glycemia |
| Decrease insulinemia |
| Increase food intake |
| Increase body weight and adiposity percentage |
| Increase GH secretion |
In summary, there are multiple studies suggesting that ghrelin could have an important function in glucose homeostasis and insulin release and probably insulin action. At least theoretically ghrelin and/or its signalling manipulation could be used for the treatment or prevention of diseases of glucose homeostasis such as type 2 diabetes.
Acknowledgment
The work is supported in part by FIS del Instituto de Salud Carlos III PI051024, PI070413, Red de Grupos RGTO (G03/028, PI050983), and Xunta de Galicia PS07/12, PGIDT05PXIC91605PN, INCITE08ENA916110ES, and Redes 2006/27, Spain.
References
- 1.Muller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiological Reviews. 1999;79(2):511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- 2.Bowers CY. Growth hormone-releasing peptide (GHRP) Cellular and Molecular Life Sciences. 1998;54(12):1316–1329. doi: 10.1007/s000180050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith RG. Development of growth hormone secretagogues. Endocrine Reviews. 2005;26(3):346–360. doi: 10.1210/er.2004-0019. [DOI] [PubMed] [Google Scholar]
- 4.Smith RG, Cheng K, Schoen WR, et al. A nonpeptidyl growth hormone secretagogue. Science. 1993;260(5114):1640–1643. doi: 10.1126/science.8503009. [DOI] [PubMed] [Google Scholar]
- 5.Smith RG, Leonard R, Bailey ART, et al. Growth hormone secretagogue receptor family members and ligands. Endocrine. 2001;14(1):9–14. doi: 10.1385/ENDO:14:1:009. [DOI] [PubMed] [Google Scholar]
- 6.Smith RG, Sun Y, Betancourt L, Asnicar M. Growth hormone secretagogues: prospects and potential pitfalls. Best Practice and Research: Clinical Endocrinology and Metabolism. 2004;18(3):333–347. doi: 10.1016/j.beem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Isidro ML, Cordido F. Growth hormone secretagogues. Combinatorial Chemistry and High Throughput Screening. 2006;9(3):175–180. doi: 10.2174/138620706776055458. [DOI] [PubMed] [Google Scholar]
- 8.Smith RG, Feighner S, Prendergast K, Guan X, Howard A. A new orphan receptor involved in pulsatile growth hormone release. Trends in Endocrinology and Metabolism. 1999;10(4):128–135. doi: 10.1016/s1043-2760(98)00132-5. [DOI] [PubMed] [Google Scholar]
- 9.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 10.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 11.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 12.van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocrine Reviews. 2004;25(3):426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 13.Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 14.Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor—identification of a potent inverse agonist. Molecular Endocrinology. 2003;17(11):2201–2210. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- 15.Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. Journal of Clinical Endocrinology and Metabolism. 2002;87(6):2988–2991. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(17):6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Davenport AP, Bonner TI, Foord SM, et al. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacological Reviews. 2005;57(4):541–546. doi: 10.1124/pr.57.4.1. [DOI] [PubMed] [Google Scholar]
- 19.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochemical and Biophysical Research Communications. 2000;279(3):909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Castro P, Isidro ML, Garcia-Buela J, et al. Marked GH secretion after ghrelin alone or combined with GH-releasing hormone (GHRH) in obese patients. Clinical Endocrinology. 2004;61(2):250–255. doi: 10.1111/j.1365-2265.2004.02092.x. [DOI] [PubMed] [Google Scholar]
- 21.Baldanzi G, Filigheddu N, Cutrupi S, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. Journal of Cell Biology. 2002;159(6):1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filigheddu N, Gnocchi VF, Coscia M, et al. Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Molecular Biology of the Cell. 2007;18(3):986–994. doi: 10.1091/mbc.E06-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nonogaki K, Ohashi-Nozue K, Oka Y. Induction of hypothalamic serum- and glucocorticoid-induced protein kinase-1 gene expression and its relation to plasma des-acyl ghrelin in energy homeostasis in mice. Biochemical and Biophysical Research Communications. 2006;344(2):696–699. doi: 10.1016/j.bbrc.2006.03.196. [DOI] [PubMed] [Google Scholar]
- 24.Sato M, Nakahara K, Goto S, et al. Effects of ghrelin and des-acyl ghrelin on neurogenesis of the rat fetal spinal cord. Biochemical and Biophysical Research Communications. 2006;350(3):598–603. doi: 10.1016/j.bbrc.2006.09.088. [DOI] [PubMed] [Google Scholar]
- 25.Toshinai K, Yamaguchi H, Sun Y, et al. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147(5):2306–2314. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Chai B, Li J-Y, Wang H, Mulholland MW. Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology. 2008;149(9):4710–4716. doi: 10.1210/en.2008-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ariyasu H, Takaya K, Iwakura H, et al. Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology. 2005;146(1):355–364. doi: 10.1210/en.2004-0629. [DOI] [PubMed] [Google Scholar]
- 28.Thompson NM, Gill DA, Davies R, et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145(1):234–242. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- 29.Delhanty PJD, van der Eerden BCJ, van der Velde M, et al. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. Journal of Endocrinology. 2006;188(1):37–47. doi: 10.1677/joe.1.06404. [DOI] [PubMed] [Google Scholar]
- 30.Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. Journal of Clinical Endocrinology and Metabolism. 2001;86(10):4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 31.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 32.Tschöp M, Wawarta R, Riepl RL, et al. Post-prandial decrease of circulating human ghrelin levels. Journal of Endocrinological Investigation. 2001;24(6):RC19–RC21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 33.Longo KA, Charoenthongtrakul S, Giuliana DJ, et al. Improved insulin sensitivity and metabolic flexibility in ghrelin receptor knockout mice. Regulatory Peptides. 2008;150(1–3):55–61. doi: 10.1016/j.regpep.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149(2):843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Molecular and Cellular Biology. 2003;23(22):7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metabolism. 2006;3(5):379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Pfluger PT, Kirchner H, Gunnel S, et al. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. American Journal of Physiology. 2008;294(3):G610–G618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- 38.Asakawa A, Inui A, Fujimiya M, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54(1):18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwakura H, Hosoda K, Son C, et al. Analysis of rat insulin II promoter-ghrelin transgenic mice and rat glucagon promoter-ghrelin transgenic mice. Journal of Biological Chemistry. 2005;280(15):15247–15256. doi: 10.1074/jbc.M411358200. [DOI] [PubMed] [Google Scholar]
- 40.Chapman IM, Bach MA, Van Cauter E, et al. Stimulation of the growth hormone (GH)-insulin-like growth factor I axis by daily oral administration of a GH secretogogue (MK-677) in healthy elderly subjects. Journal of Clinical Endocrinology and Metabolism. 1996;81(12):4249–4257. doi: 10.1210/jcem.81.12.8954023. [DOI] [PubMed] [Google Scholar]
- 41.Chapman IM, Hartman ML, Pezzoli SS, Thorner MO. Enhancement of pulsatile growth hormone secretion by continuous infusion of a growth hormone-releasing peptide mimetic, L-692,429, in older adults—a clinical research center study. Journal of Clinical Endocrinology and Metabolism. 1996;81(8):2874–2880. doi: 10.1210/jcem.81.8.8768844. [DOI] [PubMed] [Google Scholar]
- 42.Clark RG, Thomas GB, Mortensen DL, et al. Growth hormone secretagogues stimulate the hypothalamic-pituitary-adrenal axis and are diabetogenic in the Zucker diabetic fatty rat. Endocrinology. 1997;138(10):4316–4323. doi: 10.1210/endo.138.10.5424. [DOI] [PubMed] [Google Scholar]
- 43.Muller AF, Janssen JA, Hofland LJ, et al. Blockade of the growth hormone (GH) receptor unmasks rapid GH-releasing peptide-6-mediated tissue-specific insulin resistance. Journal of Clinical Endocrinology and Metabolism. 2001;86(2):590–593. doi: 10.1210/jcem.86.2.7173. [DOI] [PubMed] [Google Scholar]
- 44.Davidson MB. Effect of growth hormone on carbohydrate and lipid metabolism. Endocrine Reviews. 1987;8(2):115–131. doi: 10.1210/edrv-8-2-115. [DOI] [PubMed] [Google Scholar]
- 45.Ameen C, Linden D, Larsson B-M, Mode A, Holmang A, Oscarsson J. Effects of gender and GH secretory pattern on sterol regulatory element-binding protein-1c and its target genes in rat liver. American Journal of Physiology. 2004;287(6):E1039–E1048. doi: 10.1152/ajpendo.00059.2004. [DOI] [PubMed] [Google Scholar]
- 46.Van der Lely AJ. Justified and unjustified use of growth hormone. Postgraduate Medical Journal. 2004;80(948):577–580. doi: 10.1136/pgmj.2003.017467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts TJ, Azain MJ, Hausman GJ, Martin RJ. Interaction of insulin and somatotropin on body weight gain, feed intake, and body composition in rats. American Journal of Physiology. 1994;267(2, part 1):E293–E299. doi: 10.1152/ajpendo.1994.267.2.E293. [DOI] [PubMed] [Google Scholar]
- 48.Bengtsson B-A, Eden S, Lonn L, et al. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. Journal of Clinical Endocrinology and Metabolism. 1993;76(2):309–317. doi: 10.1210/jcem.76.2.8432773. [DOI] [PubMed] [Google Scholar]
- 49.Murray RD, Adams JE, Shalet SM. Adults with partial growth hormone deficiency have an adverse body composition. Journal of Clinical Endocrinology and Metabolism. 2004;89(4):1586–1591. doi: 10.1210/jc.2003-030761. [DOI] [PubMed] [Google Scholar]
- 50.Beshyah SA, Freemantle C, Thomas E, et al. Abnormal body composition and reduced bone mass in growth hormone deficient hypopituitary adults. Clinical Endocrinology. 1995;42(2):179–189. doi: 10.1111/j.1365-2265.1995.tb01860.x. [DOI] [PubMed] [Google Scholar]
- 51.Balbis A, Bartke A, Turyn D. Overexpression of bovine growth hormone in transgenic mice is associated with changes in hepatic insulin receptors and in their kinase activity. Life Sciences. 1996;59(16):1363–1371. doi: 10.1016/0024-3205(96)00462-6. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology. 2007;148(6):2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
- 53.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Hormone and IGF Research. 2004;14(4):309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Date Y, Nakazato M, Hashiguchi S, et al. Ghrelin is present in pancreatic α-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51(1):124–129. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 55.Volante M, Allia E, Gugliotta P, et al. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. Journal of Clinical Endocrinology and Metabolism. 2002;87(3):1300–1308. doi: 10.1210/jcem.87.3.8279. [DOI] [PubMed] [Google Scholar]
- 56.Colombo M, Gregersen S, Xiao J, Hermansen K. Effects of ghrelin and other neuropeptides (CART, MCH, orexin A and B, and GLP-1) on the release of insulin from isolated rat islets. Pancreas. 2003;27(2):161–166. doi: 10.1097/00006676-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. Journal of Histochemistry and Cytochemistry. 2004;52(3):301–310. doi: 10.1177/002215540405200301. [DOI] [PubMed] [Google Scholar]
- 58.Dezaki K, Hosoda H, Kakei M, et al. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: implication in the glycemic control in rodents. Diabetes. 2004;53(12):3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 59.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regulatory Peptides. 2002;107(1–3):63–69. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 60.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing β cells in two mouse models of pancreas development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):2924–2929. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochemical and Biophysical Research Communications. 2001;280(3):904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- 62.Masuda Y, Tanaka T, Inomata N, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochemical and Biophysical Research Communications. 2000;276(3):905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 63.Latour MG, Lautt WW. The hepatic vagus nerve in the control of insulin sensitivity in the rat. Autonomic Neuroscience. 2002;95(1-2):125–130. doi: 10.1016/s1566-0702(01)00390-3. [DOI] [PubMed] [Google Scholar]
- 64.Matsuhisa M, Yamasaki Y, Shiba Y, et al. Important role of the hepatic vagus nerve in glucose uptake and production by the liver. Metabolism. 2000;49(1):11–16. doi: 10.1016/s0026-0495(00)90538-9. [DOI] [PubMed] [Google Scholar]
- 65.Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural gh secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. Journal of Clinical Endocrinology and Metabolism. 2001;86(10):5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 66.Broglio F, Benso A, Gottero C, et al. Non-acylated ghrelin does not possess the pituitaric and pancreatic endocrine activity of acylated ghrelin in humans. Journal of Endocrinological Investigation. 2003;26(3):192–196. doi: 10.1007/BF03345156. [DOI] [PubMed] [Google Scholar]
- 67.Broglio F, Gottero C, Prodam F, et al. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. Journal of Clinical Endocrinology and Metabolism. 2004;89(6):3062–3065. doi: 10.1210/jc.2003-031964. [DOI] [PubMed] [Google Scholar]
- 68.Arosio M, Ronchi CL, Gebbia C, Cappiello V, Beck-Peccoz P, Peracchi M. Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels. Journal of Clinical Endocrinology and Metabolism. 2003;88(2):701–704. doi: 10.1210/jc.2002-021161. [DOI] [PubMed] [Google Scholar]
- 69.Nieminen P, Mustonen A-M. Effects of peripheral ghrelin on the carbohydrate and lipid metabolism of the tundra vole (Microtus oeconomus) General and Comparative Endocrinology. 2004;138(2):182–187. doi: 10.1016/j.ygcen.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Cui C, Ohnuma H, Daimon M, et al. Ghrelin infused into the portal vein inhibits glucose-stimulated insulin secretion in Wistar rats. Peptides. 2008;29(7):1241–1246. doi: 10.1016/j.peptides.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 71.Vestergaard ET, Djurhuus CB, Gjedsted J, et al. Acute effects of ghrelin administration on glucose and lipid metabolism. Journal of Clinical Endocrinology and Metabolism. 2008;93(2):438–444. doi: 10.1210/jc.2007-2018. [DOI] [PubMed] [Google Scholar]
- 72.Alvarez-Castro P, Isidro ML, Garcia-Buela J, Dieguez C, Casanueva FF, Cordido F. Effect of acute ghrelin administration on glycaemia and insulin levels in obese patients. Diabetes, Obesity and Metabolism. 2006;8(5):555–560. doi: 10.1111/j.1463-1326.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 73.Murata M, Okimura Y, Iida K, et al. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. Journal of Biological Chemistry. 2002;277(7):5667–5674. doi: 10.1074/jbc.M103898200. [DOI] [PubMed] [Google Scholar]
- 74.Papotti M, Ghe C, Cassoni P, et al. Growth hormone secretagogue binding sites in peripheral human tissues. Journal of Clinical Endocrinology and Metabolism. 2000;85(10):3803–3807. doi: 10.1210/jcem.85.10.6846. [DOI] [PubMed] [Google Scholar]
- 75.Asakawa A, Inui A, Kaga T, et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52(7):947–952. doi: 10.1136/gut.52.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perseghin G, Caumo A, Caloni M, Testolin G, Luzi L. Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in nonobese individuals. Journal of Clinical Endocrinology and Metabolism. 2001;86(10):4776–4781. doi: 10.1210/jcem.86.10.7902. [DOI] [PubMed] [Google Scholar]
- 77.Vestergaard ET, Hansen TK, Gormsen LC, et al. Constant intravenous ghrelin infusion in healthy young men: clinical pharmacokinetics and metabolic effects. American Journal of Physiology. 2007;292(6):E1829–E1836. doi: 10.1152/ajpendo.00682.2006. [DOI] [PubMed] [Google Scholar]
- 78.Vestergaard ET, Gormsen LC, Jessen N, et al. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes. 2008;57(12):3205–3210. doi: 10.2337/db08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sudo T, Ishiyama K, Takemoto M, et al. Pancreatic endocrine function after total gastrectomy and truncal vagotomy. American Journal of Surgery. 1982;144(5):539–544. doi: 10.1016/0002-9610(82)90576-1. [DOI] [PubMed] [Google Scholar]
- 80.Damjanovic SS, Lalic NM, Pesko PM, et al. Acute effects of ghrelin on insulin secretion and glucose disposal rate in gastrectomized patients. Journal of Clinical Endocrinology and Metabolism. 2006;91(7):2574–2581. doi: 10.1210/jc.2005-1482. [DOI] [PubMed] [Google Scholar]
- 81.Broglio F, Gottero C, Benso A, et al. Effects of ghrelin on the insulin and glycemic responses to glucose, arginine, or free fatty acids load in humans. The Journal of clinical endocrinology and metabolism. 2003;88(9):4268–4272. doi: 10.1210/jc.2002-021940. [DOI] [PubMed] [Google Scholar]
- 82.Gauna C, Meyler FM, Janssen JAMJL, et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. Journal of Clinical Endocrinology and Metabolism. 2004;89(10):5035–5042. doi: 10.1210/jc.2004-0363. [DOI] [PubMed] [Google Scholar]
- 83.Gauna C, Kiewiet RM, Janssen JAMJL, et al. Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditions. American Journal of Physiology. 2007;293(3):E697–E704. doi: 10.1152/ajpendo.00219.2007. [DOI] [PubMed] [Google Scholar]
- 84.Reimer MK, Pacini G, Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144(3):916–921. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- 85.Barazzoni R, Bosutti A, Stebel M, et al. Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. American Journal of Physiology. 2005;288(1):E228–E235. doi: 10.1152/ajpendo.00115.2004. [DOI] [PubMed] [Google Scholar]
- 86.Barazzoni R, Zanetti M, Cattin MR, et al. Ghrelin enhances in vivo skeletal muscle but not liver AKT signaling in rats. Obesity. 2007;15(11):2614–2623. doi: 10.1038/oby.2007.313. [DOI] [PubMed] [Google Scholar]
- 87.Theander-Carrillo C, Wiedmer P, Cettour-Rose P, et al. Ghrelin action in the brain controls adipocyte metabolism. Journal of Clinical Investigation. 2006;116(7):1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50(11):2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 89.Stevanović D, Nešić D, Milošević V, Starčević V, Severs WB. Consummatory behavior and metabolic indicators after central ghrelin injections in rats. Regulatory Peptides. 2008;147(1–3):52–59. doi: 10.1016/j.regpep.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 90.Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. European Journal of Endocrinology. 2002;146(2):241–244. doi: 10.1530/eje.0.1460241. [DOI] [PubMed] [Google Scholar]
- 91.Dezaki K, Sone H, Koizumi M, et al. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006;55(12):3486–3493. doi: 10.2337/db06-0878. [DOI] [PubMed] [Google Scholar]
- 92.Esler WP, Rudolph J, Claus TH, et al. Small-molecule Ghrelin receptor antagonists improve glucose tolerance, suppress appetite, and promote weight loss. Endocrinology. 2007;148(11):5175–5185. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- 93.Doi A, Shono T, Nishi M, Furuta H, Sasaki H, Nanjo K. IA-2β, but not IA-2, is induced by ghrelin and inhibits glucose-stimulated insulin secretion. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(4):885–890. doi: 10.1073/pnas.0502470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sussel L, Kalamaras J, Hartigan-O’Connor DJ, et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic β cells. Development. 1998;125(12):2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- 95.Wasmeier C, Hutton JC. Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. Journal of Biological Chemistry. 1996;271(30):18161–18170. doi: 10.1074/jbc.271.30.18161. [DOI] [PubMed] [Google Scholar]
- 96.Gauna C, Delhanty PJD, Hofland LJ, et al. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. Journal of Clinical Endocrinology and Metabolism. 2005;90(2):1055–1060. doi: 10.1210/jc.2004-1069. [DOI] [PubMed] [Google Scholar]
- 97.Cassoni P, Ghe C, Marrocco T, et al. Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. European Journal of Endocrinology. 2004;150(2):173–184. doi: 10.1530/eje.0.1500173. [DOI] [PubMed] [Google Scholar]
- 98.Cassoni P, Papotti M, Ghe C, et al. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. Journal of Clinical Endocrinology and Metabolism. 2001;86(4):1738–1745. doi: 10.1210/jcem.86.4.7402. [DOI] [PubMed] [Google Scholar]
- 99.Bedendi I, Alloatti G, Marcantoni A, et al. Cardiac effects of ghrelin and its endogenous derivatives des-octanoyl ghrelin and des-Gln14-ghrelin. European Journal of Pharmacology. 2003;476(1-2):87–95. doi: 10.1016/s0014-2999(03)02083-1. [DOI] [PubMed] [Google Scholar]
- 100.Muccioli G, Tschöp M, Papotti M, Deghenghi R, Heiman M, Ghigo E. Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. European Journal of Pharmacology. 2002;440(2-3):235–254. doi: 10.1016/s0014-2999(02)01432-2. [DOI] [PubMed] [Google Scholar]
- 101.Diz-Lois MT, Garcia-Buela J, Suarez F, Sangiao-Alvarellos S, Vidal O, Cordido F. Fasting and postprandial plasma ghrelin levels are decreased in patients with liver failure previous to liver transplantation. Endocrine. 2009;35(3):467–476. doi: 10.1007/s12020-009-9170-6. [DOI] [PubMed] [Google Scholar]
- 102.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 103.Wortley KE, Anderson KD, Garcia K, et al. Genetic deletion of ghrelin does decrease food intake but influences metabolic fuel preference. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8227–8232. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zigman JM, Nakano Y, Coppari R, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. Journal of Clinical Investigation. 2005;115(12):3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Egecioglu E, Bjursell M, Ljungberg A, et al. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. American Journal of Physiology. 2006;290(2):E317–E325. doi: 10.1152/ajpendo.00181.2005. [DOI] [PubMed] [Google Scholar]
- 106.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wortley KE, del Rincon J-P, Murray JD, et al. Absence of ghrelin protects against early-onset obesity. Journal of Clinical Investigation. 2005;115(12):3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang JV, Ren P-G, Avsian-Kretchmer O, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310(5750):996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 109.Kineman RD, Gahete MD, Luque RM. Identification of a mouse ghrelin gene transcipt that contains intron 2 and is regulated in the pituitary and hypothalamus in responce to metabolic stress. Journal of Molecular Endocrinology. 2007;38(5):511–521. doi: 10.1677/JME-06-0026. [DOI] [PubMed] [Google Scholar]
- 110.Holst B, Schwartz TW. Ghrelin receptor mutations—too little height and too much hunger. Journal of Clinical Investigation. 2006;116(3):637–641. doi: 10.1172/JCI27999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Poitout V, Robertson RP. An integrated view of β-cell dysfunction in type-II diabetes. Annual Review of Medicine. 1996;47:69–83. doi: 10.1146/annurev.med.47.1.69. [DOI] [PubMed] [Google Scholar]
- 112.Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proceedings of the Nutrition Society. 2004;63(2):363–368. doi: 10.1079/PNS2004349. [DOI] [PubMed] [Google Scholar]
- 113.Bewick GA, Kent A, Campbell D, et al. Mice with hyperghrelinemia are hyperphagic and glucose intolerant and have reduced leptin sensitivity. Diabetes. 2009;58(4):840–846. doi: 10.2337/db08-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reed JA, Benoit SC, Pfluger PT, Tschop MH, D’Alessio DA, Seeley RJ. Mice with chronically increased circulating ghrelin develop age-related glucose intolerance. American Journal of Physiology. 2008;294(4):E752–E760. doi: 10.1152/ajpendo.00463.2007. [DOI] [PubMed] [Google Scholar]
- 115.Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annual Review of Physiology. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- 116.O’Brien RM, Streeper RS, Ayala JE, Stadelmaier BT, Hornbuckle LA. Insulin-regulated gene expression. Biochemical Society Transactions. 2001;29, part 4:552–558. doi: 10.1042/bst0290552. [DOI] [PubMed] [Google Scholar]
- 117.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 118.Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 119.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. Journal of Biological Chemistry. 1996;271(49):31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 120.Nakae J, Biggs WH, III, Kitamura T, et al. Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nature Genetics. 2002;32(2):245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 121.Schmoll D, Walker KS, Alessi DR, et al. Regulation of glucose-6-phosphatase gene expression by protein kinase Bα and the Forkhead transcription factor FKHR: evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. Journal of Biological Chemistry. 2000;275(46):36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 122.Hall RK, Yamasaki T, Kucera T, Waltner-Law M, O’Brien R, Granner DK. Regulation of phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein-1 gene expression by insulin. The role of winged helix/Forkhead proteins. Journal of Biological Chemistry. 2000;275(39):30169–30175. doi: 10.1074/jbc.M004898200. [DOI] [PubMed] [Google Scholar]
- 123.Yoon JC, Puigserver P, Chen G, et al. Control of hepatic gluconeogenesis through the transcriptional coaotivator PGC-1. Nature. 2001;413(6852):131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 124.Puigserver P, Rhee J, Donovan J, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature. 2003;423(6939):550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 125.Hedeskov CJ. Mechanism of glucose-induced insulin secretion. Physiological Reviews. 1980;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- 126.Rutter GA. Nutrient-secretion coupling in the pancreatic islet β-cell: recent advances. Molecular Aspects of Medicine. 2001;22(6):247–284. doi: 10.1016/s0098-2997(01)00013-9. [DOI] [PubMed] [Google Scholar]
- 127.Schuit FC. Is GLUT2 required for glucose sensing? Diabetologia. 1997;40(1):104–111. doi: 10.1007/s001250050651. [DOI] [PubMed] [Google Scholar]
- 128.Valera A, Solanes G, Fernandez-Alvarez J, et al. Expression of GLUT-2 antisense RNA in β cells of transgenic mice leads to diabetes. Journal of Biological Chemistry. 1994;269(46):28543–28546. [PubMed] [Google Scholar]
- 129.Guillam M-T, Hummler E, Schaerer E, et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nature Genetics. 1997;17(3):327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- 130.Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes/Metabolism Reviews. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- 131.Bryan J, Crane A, Vila-Carriles WH, Babenko AP, Aguilar-Bryan L. Insulin secretagogues, sulfonylurea receptors and KATP channels. Current Pharmaceutical Design. 2005;11(21):2699–2716. doi: 10.2174/1381612054546879. [DOI] [PubMed] [Google Scholar]
- 132.MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic β-cells. Philosophical Transactions of the Royal Society B. 2005;360(1464):2211–2225. doi: 10.1098/rstb.2005.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.MacDonald PE, Salapatek AMF, Wheeler MB. Temperature and redox state dependence of native Kv2.1 currents in rat pancreatic β-cells. Journal of Physiology. 2003;546(3):647–653. doi: 10.1113/jphysiol.2002.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. American Journal of Physiology. 2008;295(6):E1287–E1297. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacology and Therapeutics. 2008;118(2):239–249. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 136.Dezaki K, Kakei M, Yada T. Ghrelin uses Gα i2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes. 2007;56(9):2319–2327. doi: 10.2337/db07-0345. [DOI] [PubMed] [Google Scholar]
- 137.Burns DL, Hausman SZ, Witvliet MH, Brennan MJ, Poolman JT, Manclark CR. Biochemical properties of pertussis toxin. Tokai Journal of Experimental and Clinical Medicine. 1988;13(supplement):181–185. [PubMed] [Google Scholar]
- 138.Smith RM, Charron MJ, Shah N, Lodish HF, Jarett L. Immunoelectron microscopic demonstration of insulin-stimulated translocation of glucose transporters to the plasma membrane of isolated rat adipocytes and masking of the carboxyl-terminal epitope of intracellular GLUT4. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(15):6893–6897. doi: 10.1073/pnas.88.15.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104(4):517–529. doi: 10.1016/s0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 140.Frevert EU, Kahn BB. Differential effects of constitutively active phosphatidylinositol 3-kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Molecular and Cellular Biology. 1997;17(1):190–198. doi: 10.1128/mcb.17.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hill MM, Clark SF, Tucker DF, Birnbaum MJ, James DE, Macaulay SL. A role for protein kinase Bβ/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Molecular and Cellular Biology. 1999;19(11):7771–7781. doi: 10.1128/mcb.19.11.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tremblay F, Dubois M-J, Marette A. Regulation of GLUT4 traffic and function by insulin and contraction in skeletal muscle. Frontiers in Bioscience. 2003;8:d1072–d1084. doi: 10.2741/1137. [DOI] [PubMed] [Google Scholar]
- 143.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. Journal of Clinical Investigation. 2000;106(2):165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khan AH, Pessin JE. Insulin regulation of glucose uptake: a complex interplay of intracellular signalling pathways. Diabetologia. 2002;45(11):1475–1483. doi: 10.1007/s00125-002-0974-7. [DOI] [PubMed] [Google Scholar]
- 145.Patel AD, Stanley SA, Murphy KG, et al. Ghrelin stimulates insulin-induced glucose uptake in adipocytes. Regulatory Peptides. 2006;134(1):17–22. doi: 10.1016/j.regpep.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 146.Kim MS, Yoon CY, Jang PG, et al. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Molecular Endocrinology. 2004;18(9):2291–2301. doi: 10.1210/me.2003-0459. [DOI] [PubMed] [Google Scholar]
- 147.Shepherd PR, Kahn BB. Glucose transporters and insulin action: implications for insulin resistance and diabetes mellitus. New England Journal of Medicine. 1999;341(4):248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 148.Neu A, Willasch A, Ehehalt S, Kehrer M, Hub R, Ranke MB. Diabetes incidence in children of different nationalities: an epidemiological approach to the pathogenesis of diabetes. Diabetologia. 2001;44(supplement 3):B21–B26. doi: 10.1007/pl00002948. [DOI] [PubMed] [Google Scholar]
- 149.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Journal of the American Medical Association. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 150.Ikezaki A, Hosoda H, Ito K, et al. Fasting plasma ghrelin levels are negatively correlated with insulin resistance and PAI-1, but not with leptin, in obese children and adolescents. Diabetes. 2002;51(12):3408–3411. doi: 10.2337/diabetes.51.12.3408. [DOI] [PubMed] [Google Scholar]
- 151.Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003;52(10):2546–2553. doi: 10.2337/diabetes.52.10.2546. [DOI] [PubMed] [Google Scholar]
- 152.Cordido F, Isidro ML, Nemina R, Sangiao-Alvarellos S. Ghrelin and growth hormone secretagogues, physiological and pharmacological aspect. Current Drug Discovery Technologies. 2009;6(1):34–42. doi: 10.2174/157016309787581048. [DOI] [PubMed] [Google Scholar]
- 153.Van der Lely AJ. Ghrelin and new metabolic frontiers. Hormone Research. 2009;71(supplement 1):129–133. doi: 10.1159/000178055. [DOI] [PubMed] [Google Scholar]
- 154.Isidro ML, Cordido F. Drug treatment of obesity: established and emerging therapies. Mini-Reviews in Medicinal Chemistry. 2009;9(6):664–673. doi: 10.2174/138955709788452739. [DOI] [PubMed] [Google Scholar]
- 155.Perez-Fontan M, Cordido F, Rodriguez-Carmona A, et al. Acute plasma ghrelin and leptin responses to oral feeding or intraperitoneal hypertonic glucose-based dialysate in patients with chronic renal failure. Kidney International. 2005;68(6):2877–2885. doi: 10.1111/j.1523-1755.2005.00761.x. [DOI] [PubMed] [Google Scholar]