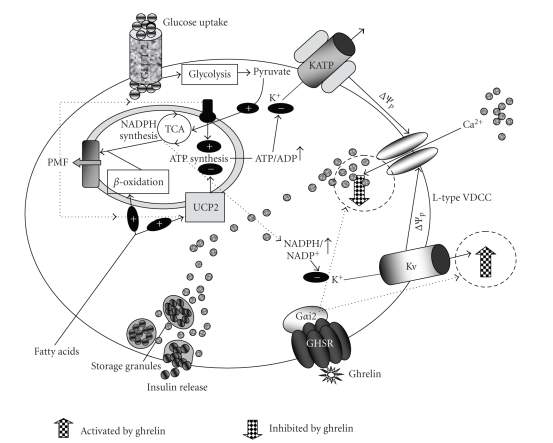
Figure 2.
β-cell mechanisms of insulin release and its regulation by ghrelin. When the plasma glucose concentration rises, β-cells oxidize it. Glucose oxidation establishes a protonmotive force (PMF) that drives ATP synthesis, increasing the ATP/ADP ratio. This causes closure of KATP-channels, depolarisation of the plasma membrane potential (Δψp) and Ca2+ flux into the cell, triggering insulin release. UCP2 activity dissipates the protonmotive force, lowering ATP/ADP. Ghrelin directly acts on the β-cell and via PTX–sensitive mechanisms attenuates glucose-induced [Ca2+]i signalling partly through enhancement of TEA-sensitive delayed outward K+ currents resulting in decrease plasma insulin levels. PTX catalyzes the ADP-ribosylation of the α subunits of the heterotrimeric G proteins Gi, Go, and Gt. This prevents the G proteins from interacting with G protein-coupled receptors on the cell membrane thus interfering with intracellular communication. Since the Gα subunits remain in their GDP-bound, inactive state, they are unable to inhibit adenylyl cyclase, thus keeping levels of adenylyl cyclase and cAMP elevated. PTX inhibited a number of insulin-stimulated cellular events, such as glucose transport and its metabolism. Antisense oligonucleotide specific for Gαi2-subunit of G proteins blocks the effects of ghrelin on [Ca2+]i and insulin release. Hence ghrelin presumably suppresses glucose-induced insulin release via Gαi2- and Kv channel–mediated attenuation of Ca2+ signalling in β-cells.