Figure 6. SIRT1 deacetylates RARβ.
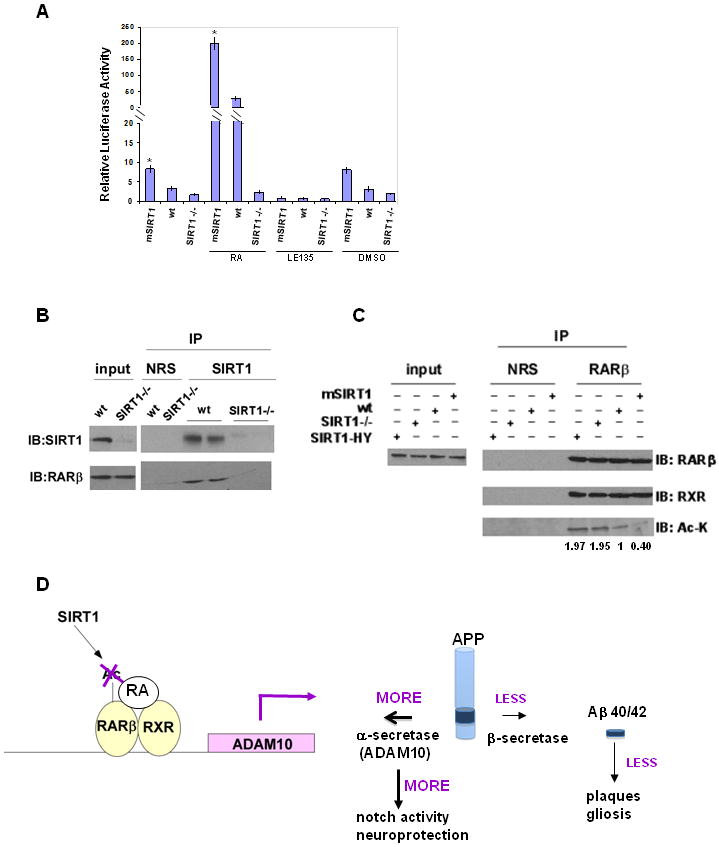
(A) Luciferase reporter assays of MEFs overexpressing SIRT1 (mSIRT1), lacking SIRT1 (SIRT1-/-) or controls (wt). Cells were transfected with luciferase reporter driven by the RARE (Retinoic Acid Receptor Element). 24 hr later, cells were incubated with RA (Retinoic Acid), LE-135 or DMSO for an additional 24 hr and assayed as described in Experimental Procedures. (B) Cell lysates from wild type (wt) and SIRT1-/- (SIRT1-/-) MEFs immunoprecipitated with normal rabbit serum (NRS) or anti-SIRT1 antibody and blotted with anti-SIRT1 and anti-RARβ antibodies. The two proteins are shown to interact at endogenous levels. (C) Cell lysates from wild type (wt), SIRT1-overxpressing (mSIRT1) MEFs, SIRT1-/- MEFs and SIRT1-/- MEFs overexpressing SIRT1 catalytic mutant HY were subjected to immunoprecipitation with anti-RARβ antibody or NRS. Immunoprecipitates were analyzed by western blotting with anti-RARβ, anti-RXR or anti-pan acetylated lysine (Ac-K) antibodies. Quantification of the acetylation levels were shown below. (see also Figure S5) (D) A model for the role of SIRT1 in suppressing Aβ production and activating notch pathway. SIRT1 deacetylates RARβ, causes RA to bind to RARβ-RXR heterodimer which binds to ADAM10 promoter. ADAM10 transcription and protein level is increased and APP processing is directed towards α-secretase pathway. This will lead to less β-secretase cleavage of APP resulting in less Aβ production, fewer β-amyloid plaques and less gliosis. ADAM10 activation by SIRT1 also induces the Notch pathway, which is known to repair neuronal damage in brain, providing neuroprotection.
