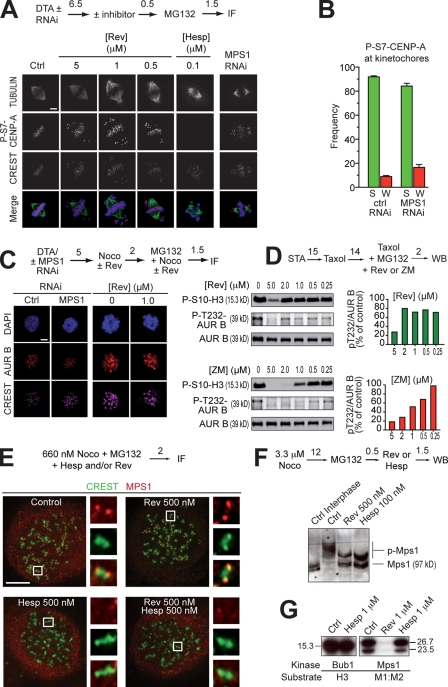
Figure 4.
MPS1 acts downstream from Aurora B. (A) P-S7–CENP-A in mitotic HeLa cells is unaltered even at 5 µM reversine (Rev). The antigen is present on centromeres/kinetochores of chromosomes near the poles, as well as of chromosomes at the equator. The antigen is invisible in the presence of 100 nM hesperadin (Hesp). No compensation from Aurora A was observed (Fig. S4 B). (B) A quantification of the results in A. “S” and “W” indicate strong and weak binding, respectively. These criteria are indicated in Fig. S4 A. Error bars are mean ± SEM. (C) Kinetochore localization of AURORA B (AUR B) in HeLa cells is unaffected after MPS1 RNAi or the addition of reversine. (D, top) Phosphorylation of the activation loop of AURORA B (P-T232) is not affected by reversine until above 2 µM. The pattern of loss of activation loop phosphorylation follows the pattern of loss of P-S10-H3 phosphorylation. (bottom) The same experiment with ZM447439 (ZM) as a positive control. (E) Kinetochore localization of MPS1 in 660 nM nocodazole (Noco) is enhanced by 0.5 µM reversine. If AURORA B is inhibited with 0.5 µM hesperadin, reversine-induced localization of MPS1 is abrogated. Images were taken on a Delta Vision microscope. The insets represent 10× zooms of the boxed areas interpolated using SoftWoRx. (F) Both MPS1 and AURORA B inhibitors reduce the phosphorylation of mitotic MPS1, as visualized through the PHOS tag method (Kinoshita et al., 2006). (A and C–F) Numbers above arrows indicate time in hours. (G) Hesperadin does not inhibit BUB1 or MPS1 in an in vitro kinase assay (see also Table S1). Molecular mass is indicated in kilodaltons. Ctrl, control; DTA, double thymidine arrest; IF, immunofluorescence; WB, Western blot. Bars, 5 µm.