Summary
Background
Results of several trials of antioxidant use during pregnancy have not shown a reduction in pre-eclampsia, but the effect in women with diabetes is unknown. We aimed to assess whether supplementation with vitamins C and E reduced incidence of pre-eclampsia in women with type 1 diabetes.
Methods
We enrolled women from 25 UK antenatal metabolic clinics in a multicentre randomised placebo-controlled trial. Eligibility criteria were type 1 diabetes preceding pregnancy, presentation between 8 weeks' and 22 weeks' gestation, singleton pregnancy, and age 16 years or older. Women were randomly allocated in a 1:1 ratio to receive 1000 mg vitamin C and 400 IU vitamin E (α-tocopherol) or matched placebo daily until delivery. The randomisation sequence was stratified by centre with balanced blocks of eight patients. All trial personnel and participants were masked to treatment allocation. The primary endpoint was pre-eclampsia, which we defined as gestational hypertension with proteinuria. Analysis was by modified intention to treat. This study is registered, ISRCTN27214045.
Findings
Between April, 2003, and June, 2008, 762 women were randomly allocated to treatment groups (379 vitamin supplementation, 383 placebo). The primary endpoint was assessed for 375 women allocated to receive vitamins, and 374 allocated to placebo. Rates of pre-eclampsia did not differ between vitamin (15%, n=57) and placebo (19%, 70) groups (risk ratio 0·81, 95% CI 0·59–1·12). No adverse maternal or neonatal outcomes were reported.
Interpretation
Supplementation with vitamins C and E did not reduce risk of pre-eclampsia in women with type 1 diabetes. However, the possibility that vitamin supplementation might be beneficial in women with a low antioxidant status at baseline needs further testing.
Funding
The Wellcome Trust.
Introduction
Pre-eclampsia is a multisystem disorder of pregnancy that is characterised by pregnancy-induced or gestational hypertension and new-onset proteinuria during the second half of pregnancy.1 Recognised risk factors are nulliparity, age younger than 20 years or older than 40 years, obesity, history of pre-eclampsia, multiple pregnancy, and pre-existing disorders such as chronic hypertension, renal disease, autoimmune disease, antiphospholipid syndrome, and diabetes mellitus.2 Pre-eclampsia results in serious maternal complications such as eclampsia and HELLP (haemolysis, elevated liver enzymes and low platelets) syndrome, and is a foremost cause of maternal death.3 Moreover, since delivery is the only cure, up to 15% of preterm births are associated with pre-eclampsia, with a consequent increase in infant mortality and morbidity.4 The global prevalence is around 4%,3 but the rate is raised two to four times in women with type 1 diabetes, and increases with complexity of diabetes.5–7
The hypothesis that oxidative stress plays a key part in pathogenesis of pre-eclampsia was proposed in the late 1980s,8 and has since been the focus of much research.9,10 Diabetes mellitus, specifically type 1 diabetes, is associated with increased oxidative stress and antioxidant depletion,11,12 which is partly related to prevailing blood glucose concentrations.13 Increased oxidative stress in pregnant women with diabetes14,15 might account for rates of pre-eclampsia that are two to four times higher in this group, especially in those with diabetes-related complications,5–7 and lends support to the postulated role of oxidative stress in pathophysiology of the disorder in diabetes-associated pregnancy.16
In a small randomised placebo-controlled trial,17 supplementation with vitamins C and E was associated with a reduction in rate of pre-eclampsia from 17% to 8% (adjusted odds ratio 0·39, 95% CI 0·17–0·90) in 283 women at high risk of developing the disorder. These results added to existing evidence for a role of oxidative stress in pathogenesis of pre-eclampsia, and led to several large trials of antioxidant treatment for prevention of pre-eclampsia.18–24 The results of these trials have shown no benefit of vitamin C and E supplementation during pregnancy;18,19,21–24 however, only three included women with diabetes, and in each instance these groups were small and poorly characterised.18,19,23 Since pre-eclampsia is likely to be a heterogeneous disease,9 pathogenesis could vary between women with different risk factors. Furthermore, in view of the increase in oxidative stress and antioxidant depletion that occur in diabetes, a beneficial effect of antioxidant supplementation is plausible in this group of patients.
We designed the Diabetes and Pre-eclampsia Intervention Trial (DAPIT) to assess whether supplementation with vitamins C and E reduced incidence of pre-eclampsia in women with type 1 diabetes.20
Methods
Study design and patients
DAPIT was a multicentre, randomised, placebo-controlled, parallel-group trial. Women were recruited from 25 antenatal metabolic clinics across Northern Ireland, Scotland, and northwest England between April, 2003, and June, 2008. The last baby was delivered in December, 2008. Eligibility criteria were type 1 diabetes preceding pregnancy, presentation between 8 weeks' and 22 weeks' gestation, singleton pregnancy, and age 16 years or older. Women were excluded if they did not give consent, were enrolled in another research study, were being treated with warfarin, or were known to misuse drugs. Women taking vitamin supplements were excluded only if these contained 500 mg or more vitamin C or 200 IU or more vitamin E daily. Women with chronic hypertension were included in the trial. The West Midlands multicentre research ethics committee provided ethics approval (MREC 02/7/016). Participants gave written informed consent and had at least 48 h to review the patient information sheet.
Randomisation and masking
Participants were randomly allocated in a 1:1 ratio to receive 1000 mg vitamin C and 400 IU vitamin E or matched placebo daily from between 8 and 22 weeks' gestation until delivery. Vitamin C and identical placebo (calcium carbonate, microcrystalline cellulose, maltrodextrin, and stearic acid) tablets were manufactured by Thompson & Capper (Astmoor, Runcorn, Cheshire, UK). Natural-source vitamin E (α-tocopherol) and identical placebo (olive oil) capsules were manufactured by Eurocaps Limited (Dukestown, Tredegar, Gwent, UK). Victoria Pharmaceuticals (The Royal Hospitals, Belfast, UK) packaged tablets and capsules, 120 per bottle, according to a randomisation sequence generated in advance by Victoria Pharmaceuticals using PRISYM ID software (version 1.0009). The randomisation sequence was stratified by centre with balanced blocks of eight patients, and was held by Victoria Pharmaceuticals. Individual sealed envelopes containing treatment allocations were given to trial pharmacists in every centre, allowing treatment group to be revealed in a clinical emergency. Treatment allocation was masked from all trial personnel and participants until trial completion.
Procedures
Eligible women who gave consent were assigned the next available number at that centre by research midwives, and given their first supply of trial drugs—bottles of vitamin C tablets and vitamin E capsules (vitamin group) or bottles of placebos (placebo group)—along with a 7-day pill dispenser to aid adherence. Participants were instructed to take one tablet and one capsule daily until delivery, and to leave unused pills in the bottles. Participants attended trial visits at 26 (within 2) weeks' and 34 (within 2) weeks' gestation, at which times tablets and capsules were counted and the next supply dispensed. Unused tablets and capsules were collected during delivery admission or at the 6-week postnatal trial visit, or were returned in postage prepaid envelopes.
Blood pressure at randomisation was measured with a British Hypertension Society validated automated instrument, (Omron M5-I, Omron Healthcare, West Sussex, UK). After the participant had been seated for 5 min, the average of two measurements taken 3 min apart was recorded. Biological samples obtained at baseline, 26 weeks', and 34 weeks' gestation were batch analysed at the end of the study at the central laboratory (Queen's University, Belfast) for plasminogen activator inhibitor type 1 (PAI-1) and type 2 (PAI-2), plasma ascorbate concentrations, serum concentrations of α-tocopherol (expressed per mmol of serum cholesterol), serum total cholesterol, HbA1c, and urine microalbumin and creatinine. Laboratory analyses are detailed in the webappendix. When possible, follow-up data were obtained for study participants at routine clinic visits 6 weeks after delivery and for infants at 8 week postnatal checks with their paediatrician, family doctor, or health visitor. These data were measurement of weight, length, and head circumference and assessment of fixation, following, smiling, head control, tone, tendon reflexes, heart murmurs, and congenital abnormalities.
The primary outcome was pre-eclampsia, which we defined as gestational hypertension with proteinuria in accordance with the International Society for the Study of Hypertension in Pregnancy guidelines.1 Gestational hypertension was defined as two diastolic blood pressure readings of 90 mm Hg or more at least 4 h apart, or one reading of at least 110 mm Hg, occurring after 20 weeks' gestation or up to 48 h postnatally and excluding labour. Proteinuria was defined as a result of at least 1+ for dipstick analysis of a midstream specimen on two or more occasions or more than 300 mg urinary protein per 24 h. For women with diastolic blood pressure greater than 90 mm Hg at the first antenatal visit, superimposed pre-eclampsia was defined as the development of proteinuria and a rise of at least 10 mm Hg from the first recorded antenatal diastolic blood pressure on two occasions at least 4 h apart or one reading of at least 110 mm Hg, occurring from 20 weeks' gestation until 48 h postnatally and excluding labour. For women with pre-existing proteinuria, diagnosis was made by a doubling of proteinuria on the basis of dipstick or quantitative measurements or on clinical or biochemical grounds by identification of one additional feature of pre-eclampsia (eg, HELLP syndrome, eclampsia). Staff in the trial centre reviewed all participants with hypertension. Additionally, diagnosis was independently confirmed by three senior clinicians, who were unaware of treatment allocation.
Secondary outcomes were placental and endothelial function as established by PAI-1 to PAI-2 ratio, gestational hypertension, and birthweight centile as calculated from customised birthweight charts.25 We prespecified several additional maternal and neonatal outcomes, including miscarriage, maternal death, obstetric complications and other adverse outcomes, fetal malformation, gestational age at delivery, and admission to a neonatal care unit. We expressed growth measures (weight, length, head circumference) at birth and postnatal follow-up (6–12 weeks of age) as SD scores using the 1990 British Growth Standard.26
Statistical analysis
On the assumption of a 20% rate of pre-eclampsia, a study size of 756 women had greater than 80% power to detect a 40% reduction (from 20% to 12%) as significant (p<0·05; two-tailed). We did seven interim analyses for the primary endpoint using the Haybittle-Peto method27 and presented results to the independent data monitoring committee. Analysis of the primary endpoint was by modified intention to treat, for all pregnancies of more than 20 weeks' gestation. We undertook 11 predefined subgroup analyses for the primary endpoint: baseline control of diabetes; HbA1c less than 7%, 7–8%, and more than 8%; baseline antioxidant status (vitamin E <3 μmol/mmol cholesterol, 3–5 μmol/mmol cholesterol, and >5 μmol/mmol cholesterol; vitamin C <10 μmol/L, 10–30 μmol/L, and >30 μmol/L); and current smoking (yes or no).
Treatment group comparisons were summarised as differences in means or risk ratios (with 95% CIs) and independent samples t tests and χ2 or Fisher's exact tests were applied. To allow for multiple comparisons, we used a strict significance level (p<0·01) for secondary outcomes. Log transformations were applied in an attempt to normalise the distribution of PAI-1 to PAI-2 ratio, but comparisons were done with the Mann-Whitney U test. SPSS (version 17) was used for all analyses.
This study is registered, ISRCTN27214045.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
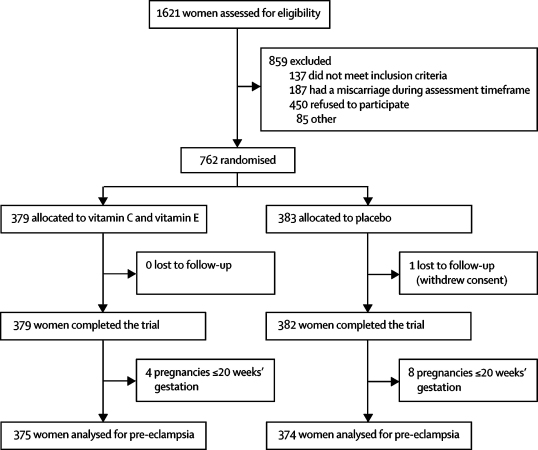
Figure 1 shows the trial profile. Of the 762 women enrolled, 379 were randomly allocated to receive vitamins C and E and 383 to placebo. Outcome data were available for 761 women (379 vitamin, 382 placebo), and 749 women were assessed for pre-eclampsia, by original assigned group (375 vitamin, 374 placebo). There were 12 deviations from the inclusion and exclusion criteria—eight women were enrolled outside the 22-week cutoff for gestation (all were within 4 days of this threshold) and four patients were later reclassified as having type 2 diabetes. All 12 women were included in the analysis.
Figure 1.
Trial profile
Although most maternal baseline characteristics did not differ between groups, history of pre-eclampsia, hypertension, antihypertensive treatment, and microalbuminuria were more common in the placebo group than in the vitamin group (table 1). On the basis of counts of returned pills after delivery (or at the 34-week visit if no count was available after delivery, n=45) from 618 women, 524 (85%) took at least 50% of their tablets, 434 (70%) took 80% or more, and 237 (38%) took all their tablets; 17 (3%) did not take any. Estimated percentage adherence did not differ between groups (vitamin C, median 95% [IQR 75–100] vs placebo, 96% [74–100]; vitamin E, 93% [78–100] vs placebo, 93% [74–100]).
Table 1.
Baseline maternal characteristics
| Vitamins C and E (N=379) | Placebo (N=383) | ||
|---|---|---|---|
| Age (years) | 29·5 (5·6) | 29·6 (5·7) | |
| Gestation (weeks) | 14·3 (3·6) | 14·2 (3·4) | |
| Body-mass index (kg/m2) | 27·6 (5·4) | 27·4 (4·6) | |
| Ethnic origin | |||
| White | 364 (96%) | 371 (97%) | |
| Black | 3 (1%) | 4 (1%) | |
| Asian | 6 (2%) | 5 (1%) | |
| Other/not known | 6 (2%) | 3 (1%) | |
| 12 years or fewer in full-time education | 140 (37%) | 159 (42%) | |
| Primigravida | 190 (50%) | 188 (49%) | |
| Blood pressure (mm Hg) | |||
| Systolic | 119·2 (12·2) | 118·4 (11·6) | |
| Diastolic | 74·6 (8·7) | 74·5 (8·4) | |
| Antihypertensive treatment before this pregnancy | 21 (6%) | 40 (10%) | |
| History of hypertension before pregnancy | 46 (12%) | 66 (17%) | |
| History of pre-eclampsia in previous pregnancy | 6 (2%) | 20 (5%) | |
| Diabetes | |||
| Duration (years) | 14·0 (8·3) | 15·0 (8·0) | |
| HbA1c at randomisation (%) | 7·2 (0·9) | 7·2 (1·0) | |
| Total daily insulin dose at randomisation (units) | 58 (44–73) | 56 (43–72) | |
| Renal status before this pregnancy | |||
| Normal | 355 (94%) | 338 (88%) | |
| Microalbuminuria | 13 (3%) | 23 (6%) | |
| Macroalbuminuria | 1 (<1%) | 3 (1%) | |
| 24-h urinary protein more than 3 g per 24 h | 0 | 4 (1%) | |
| Not known | 10 (3%) | 15 (4%) | |
| Albumin-to-creatinine ratio at randomisation (mg/mmol) | 0·71 (0·38–1·28) | 0·73 (0·41–1·95) | |
| Current smoker | 75 (20%) | 74 (19%) | |
| Taking aspirin at randomisation | 28 (7%) | 31 (8%) | |
| Taking multivitamin supplements at randomisation | 38 (10%) | 35 (9%) | |
Data are mean (SD), number (%), or median (IQR).
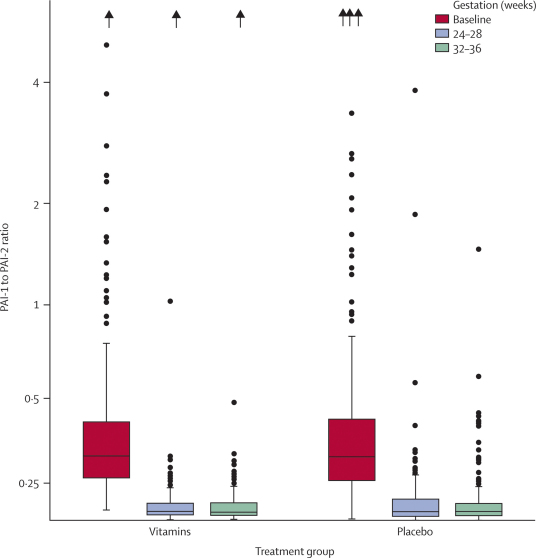
Overall, 127 (17%) women developed pre-eclampsia. Risk of pre-eclampsia did not differ between vitamin and placebo groups (table 2). There were no significant differences between groups in risk of gestational hypertension or birthweight lower than the tenth centile for gestational age (table 2). PAI-1 to PAI-2 ratios did not differ between groups at baseline (p=0·40), 26 weeks (p=0·32), and 34 weeks (p=0·78) (figure 2).
Table 2.
Primary and secondary clinical outcomes
| Vitamins C and E (N=379) | Placebo (N=382) | Risk ratio (95% CI) | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Pre-eclampsia* | 57/375 (15%) | 70/374 (19%) | 0·81 (0·59–1·12) | 0·20 |
| Secondary outcomes | ||||
| Gestational hypertension* | 42/375 (11%) | 41/374 (11%) | 1·02 (0·68–1·53) | 0·92 |
| Birthweight lower than the tenth centile for gestational age25 | 23/373 (6%) | 36/372 (10%) | 0·64 (0·39–1·05) | 0·08 |
Data are n/N (%) or risk ratio (95% CI).
Analysis included all pregnancies greater than 20 weeks' gestational age.
Figure 2.
PAI-1 to PAI-2 ratio by weeks' gestation
PAI-1 to PAI-2 ratio in vitamin and placebo groups at baseline, 26 weeks', and 34 weeks' gestation (within 2 weeks). Boxes show IQRs, and medians are shown by horizontal lines. Vertical lines extend to the highest and lowest values, but outliers (results exceeding the upper quartile by more than 1·5 times the IQR) are identified as individual symbols. Values greater than 5 are shown by arrows at the top of the figure. PAI=plasminogen activator inhibitor.
We noted no significant differences between vitamin and placebo groups for any maternal outcome, including delivery after a hypertension-related admission before 34 or 37 weeks, but fewer babies were born preterm (<37 weeks' gestation) in the vitamin group than in the placebo group (table 3). There were no significant differences between vitamin and placebo groups for any clinical neonatal outcome including fetal malformation, fetal loss, infant death, or miscarriage. Rates of admission to neonatal care, including intensive care, were similar in both groups, as were rates of respiratory diagnoses and other complications (table 3). Table 4 shows mean birthweights for both vitamin and placebo groups; risk of birthweights of 2500 g or less (RR 0·82, 95% CI 0·56–1·20) and 4000 g or more (1·25, 0·95–1·64) did not differ between groups. No significant differences were found in SD scores for weight, length, or head circumference between infants from each group at birth or follow-up (table 4). We noted no adverse events or side effects attributable to supplementation with vitamin C or E in mothers or infants.
Table 3.
Maternal and neonatal outcomes
| Vitamins C and E (N=379) | Placebo (N=382) | Risk ratio (95% CI) | p value | ||
|---|---|---|---|---|---|
| Maternal | |||||
| Miscarriage | 4/379 (1%) | 4/382 (1%) | 1·01 (0·25–4·00) | 1·00 | |
| Elective termination | 1/379 (<1%) | 5/382 (1%) | 0·20 (0·02–1·72) | 0·22 | |
| Maternal death | 0/379 | 1/382 (<1%) | NA | 1·00 | |
| Delivery following a hypertension-related admission | |||||
| <34 weeks' gestation | 13/375 (3%) | 12/374 (3%) | 1·08 (0·50–2·34) | 0·84 | |
| <37 weeks' gestation | 43/375 (11%) | 48/374 (13%) | 0·89 (0·61–1·31) | 0·57 | |
| Obstetric complications | |||||
| Eclampsia | 1/375 (<1%) | 2/374 (1%) | 0·50 (0·05–5·48) | 0·62 | |
| HELLP syndrome | 3/375 (1%) | 2/374 (1%) | 1·50 (0·25–8·90) | 1·00 | |
| Pulmonary oedema | 1/375 (<1%) | 2/374 (1%) | 0·50 (0·05–5·48) | 0·62 | |
| Placental abruption | 5/375 (1%) | 7/374 (2%) | 0·71 (0·23–2·22) | 0·56 | |
| PPROM (<37 weeks) | 23/375 (6%) | 31/374 (8%) | 0·74 (0·44–1·24) | 0·25 | |
| Other adverse outcomes | |||||
| DBP >110 mm Hg | 13/374 (3%) | 15/375 (4%) | 0·87 (0·42–1·80) | 0·71 | |
| >5 g proteinuria per day | 9/375 (2%) | 9/373 (2%) | 0·99 (0·40–2·48) | 0·99 | |
| Platelets <100 000 per mL | 4/368 (1%) | 4/366 (1%) | 0·99 (0·25–3·95) | 1·00 | |
| Abnormal concentrations of alanine and aspartate aminotransferases | 23/368 (6%) | 33/371 (9%) | 0·70 (0·42–1·17) | 0·17 | |
| Polyhydramnios | 30/374 (8%) | 26/373 (7%) | 1·15 (0·69–1·91) | 0·59 | |
| Neonatal | |||||
| Fetal loss or infant death | |||||
| Late fetal loss | 1/379 (<1%) | 0/382 | NA | 1·00 | |
| Antepartum stillbirth | 9/379 (2%) | 8/382 (2%) | 1·13 (0·44–2·91) | 0·79 | |
| Neonatal death | 2/364 (1%) | 3/366 (1%) | 0·67 (0·11–3·99) | 1·00 | |
| Birthweight for gestational age25 | |||||
| <5th centile | 15/373 (4%) | 24/372 (6%) | 0·62 (0·33–1·17) | 0·14 | |
| >90th centile | 199/373 (53%) | 186/372 (50%) | 1·07 (0·93–1·23) | 0·36 | |
| Gestational age at delivery | |||||
| <28 weeks | 5/375 (1%) | 2/374 (1%) | 2·49 (0·49–12·8) | 0·45 | |
| <34 weeks | 35/375 (9%) | 36/374 (10%) | 0·97 (0·62–1·51) | 0·89 | |
| <37 weeks | 126/375 (34%) | 152/374 (41%) | 0·83 (0·69–1·00) | 0·046 | |
| Major fetal malformation | 12/378 (3%) | 17/382 (4%) | 0·71 (0·35–1·47) | 0·36 | |
| Admission to neonatal intensive care unit | |||||
| Overall | 197/363 (54%) | 205/365 (56%) | 0·97 (0·85–1·10) | 0·61 | |
| Level 1 (intensive care) | 28/363 (8%) | 39/365 (11%) | 0·72 (0·45–1·15) | 0·17 | |
| Level 2 (high dependency care) | 42/363 (12%) | 54/365 (15%) | 0·78 (0·54–1·14) | 0·20 | |
| Level 3 (special care in NICU) | 192/363 (53%) | 197/365 (54%) | 0·98 (0·86–1·12) | 0·77 | |
| Special care provided on postnatal ward | 243/363 (67%) | 225/365 (62%) | 1·09 (0·97–1·21) | 0·14 | |
| Assisted ventilation (endotracheal tube) | 20/364 (5%) | 25/364 (7%) | 0·80 (0·45–1·41) | 0·44 | |
| Phototherapy | 68/362 (19%) | 87/363 (24%) | 0·78 (0·59–1·04) | 0·09 | |
| Respiratory distress syndrome | 26/363 (7%) | 32/364 (9%) | 0·81 (0·50–1·34) | 0·42 | |
| Complications | |||||
| Necrotising enterocolitis | 0/362 | 3/365 (1%) | NA | 0·25 | |
| Seizures necessitating use of anticonvulsant drugs | 3/362 (1%) | 2/364 (1%) | 1·51 (0·25–8·97) | 0·69 | |
| Retinopathy of prematurity | 1/361 (<1%) | 2/365 (1%) | 0·51 (0·05–5·55) | 1·00 | |
| Bacteraemia (proven) | 6/363 (2%) | 14/364 (4%) | 0·43 (0·17–1·11) | 0·07 | |
| Chronic lung disease* | 2/363 (1%) | 5/363 (1%) | 0·40 (0·08–2·05) | 0·45 | |
Data are n/N (%) or risk ratio (95% CI). HELLP=haemolysis, elevated liver enzymes, low platelets. PPROM=preterm premature rupture of membranes. DBP=diastolic blood pressure. NICU=neonatal intensive care unit.
Chronic lung disease was defined as a need for oxygen at 36 weeks' corrected age, for infants born at less than 32 weeks' gestation, or the need for oxygen after day 28 for infants born at 32 weeks' gestation or more.
Table 4.
Growth measures at birth and at postnatal follow-up (6–12 weeks of age)
|
Vitamins C and E |
Placebo |
Mean difference (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | |||
| Birthweight (g) | 373 | 3435 (802) | 372 | 3355 (800) | 80 (−35 to 195) | 0·17 |
| Birthweight SD score | 372 | 1·37 (1·44) | 371 | 1·28 (1·51) | 0·09 (−0·12 to 0·30) | 0·39 |
| Birth length SD score | 231 | 1·30 (1·75) | 222 | 1·39 (1·51) | −0·08 (−0·38 to 0·22) | 0·60 |
| Birth head circumference SD score | 349 | 0·90 (1·25) | 338 | 0·74 (1·28) | 0·16 (−0·03 to 0·35) | 0·10 |
| Follow-up weight SD score | 292 | 0·72 (1·13) | 289 | 0·61 (1·12) | 0·11 (−0·07 to 0·30) | 0·23 |
| Follow-up length SD score | 264 | 0·71 (1·15) | 261 | 0·51 (1·19) | 0·21 (0·01 to 0·41) | 0·04 |
| Follow-up head circumference SD score | 270 | 0·62 (1·10) | 263 | 0·46 (1·29) | 0·16 (−0·04 to 0·37) | 0·12 |
Data are number, mean (SD), or mean difference (95% CI).
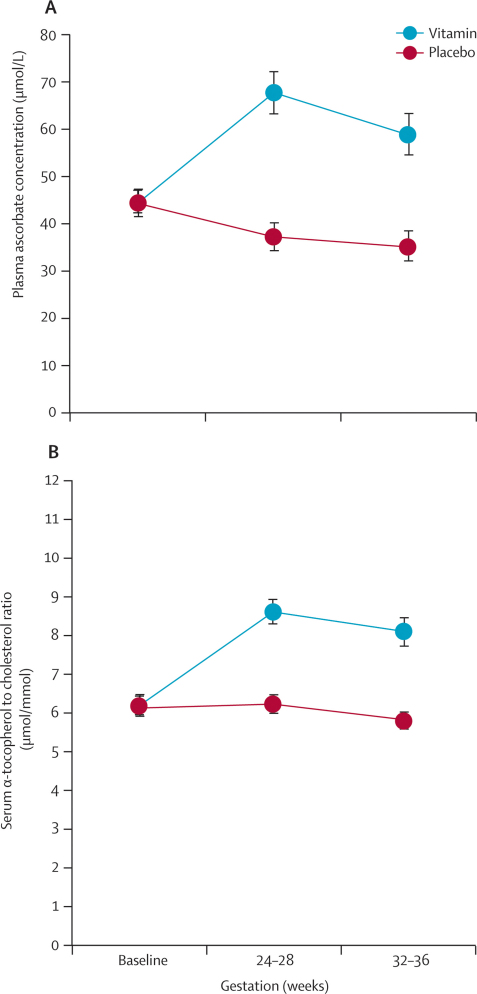
Mean plasma ascorbate concentrations (vitamin, 44·4 [SD 26·0] μmol/L vs placebo, 44·2 [26·0] μmol/L) and cholesterol-corrected serum α-tocopherol concentrations (6·15 [1·24] μmol/mmol vs 6·19 [1·20] μmol/mmol) were similar at baseline in both groups (figure 3). Blood samples were available for plasma ascorbate analysis in 590 (77%) patients at 26 weeks (299 vitamin, 291 placebo) and 511 (67%) at 34 weeks (263 vitamin, 248 placebo), and for serum α-tocopherol analysis in 614 (81%) patients at 26 weeks (309 vitamin, 305 placebo) and 536 (70%) at 34 weeks (267 vitamin, 269 placebo). Concentrations of both vitamins were significantly higher in the vitamin group than in the placebo group at 26 weeks' and 34 weeks' gestation (p<0·0001) (figure 3).
Figure 3.
Plasma ascorbate and serum α-tocopherol concentrations, by weeks' gestation
Mean plasma ascorbate (A) and serum α-tocopherol (B) concentrations in vitamin and placebo groups at baseline, 26 weeks', and 34 weeks' gestation (within 2 weeks). Error bars show 95% CIs.
Table 5 compares rates of pre-eclampsia in predefined subgroup analyses. Rates of pre-eclampsia did not vary with baseline control of diabetes or baseline smoking status. However, fewer women with baseline plasma ascorbate concentrations lower than 10 μmol/L developed pre-eclampsia in the vitamin group than in the placebo group. Furthermore, fewer women with baseline serum α-tocopherol of 3–5 μmol/mmol cholesterol developed pre-eclampsia in the vitamin group compared with placebo.
Table 5.
Rates of pre-eclampsia in 11 predefined subgroups
| Vitamins C and E | Placebo | Risk ratio (95% CI) | p value* | |
|---|---|---|---|---|
| Baseline control of diabetes | ||||
| HbA1c <7% | 18/162 (11%) | 25/156 (16%) | 0·69 (0·39–1·22) | 0·20 |
| HbA1c 7–8% | 16/115 (14%) | 23/101 (23%) | 0·61 (0·34–1·09) | 0·09 |
| HbA1c >8% | 11/49 (22%) | 15/64 (23%) | 0·96 (0·48–1·90) | 0·90 |
| Baseline vitamin C status* | ||||
| <10 μmol/L | 1/15 (7%) | 6/13 (46%) | 0·14 (0·02–0·76) | 0·03 |
| 10–30 μmol/L | 9/76 (12%) | 12/89 (13%) | 0·88 (0·39–1·97) | 0·75 |
| >30 μmol/L | 36/232 (16%) | 44/221 (20%) | 0·78 (0·52–1·16) | 0·22 |
| Baseline vitamin E status† | ||||
| <3 μmol/mmol cholesterol | 0/3 | 0/2 | .. | .. |
| 3–5 μmol/mmol cholesterol | 4/48 (8%) | 10/42 (24%) | 0·35 (0·12–0·97) | 0·04 |
| >5 μmol/mmol cholesterol | 41/281 (15%) | 55/291 (19%) | 0·77 (0·53–1·12) | 0·17 |
| Smoker at baseline | ||||
| Yes | 8/74 (11%) | 10/73 (14%) | 0·79 (0·33–1·89) | 0·59 |
| No | 49/301 (16%) | 60/301 (20%) | 0·82 (0·58–1·15) | 0·24 |
Data are n/N (%) or risk ratio (95% CI).
Plasma ascorbate concentration.
Serum α-tocopherol concentration adjusted for cholesterol.
Discussion
In this multicentre, randomised, placebo-controlled trial, daily supplementation with vitamins C and E from early to mid pregnancy did not reduce risk of pre-eclampsia, gestational hypertension, or low birthweight infants in women with type 1 diabetes, nor did it reduce the PAI-1 to PAI-2 ratio, which is a measure of endothelial activation. However, in two of 11 prespecified subgroup analyses, risk of pre-eclampsia was significantly reduced in women with low antioxidant status at baseline who were randomly allocated to the vitamin group compared with women of similar antioxidant status assigned to placebo, although the numbers were small and neither analysis was significant with the more stringent interaction test that is recommended by CONSORT.28 We noted no evidence that antioxidant supplementation was associated with any harm to mother or baby; indeed, almost all of the trends were in the direction of benefit to the supplemented group.
Several large trials of antioxidant supplementation for prevention of pre-eclampsia in women at both low22,23,24 and high risk have already been completed.18,19,21,23 All used similar doses of vitamins C and E, with women randomly allocated to treatment groups in the late first or second trimester. The findings have been uniformly negative for pre-eclampsia reported as a primary18,19,21,22 or secondary outcome.23,24 Increased rates of low birthweight,18 gestational hypertension,23,24 fetal loss,23 stillbirth18 or premature rupture of membranes21,23 were reported by investigators in some trials, but were not confirmed across all studies, and thus their significance remains uncertain. A further difficulty with some of these trials is the heterogeneous nature of the population under study.18,19,21,23 DAPIT differs from previous trials because it focused on the role of antioxidants in a homogeneous group of carefully characterised women with type 1 diabetes.
In agreement with the WHO trial,19 and by contrast with other trials,18,21–24 we noted no evidence of harm attributable to antioxidant supplementation either in mothers or infants. A Cochrane review of vitamin C supplementation in pregnancy raised concerns about a possible increased risk of preterm birth in women supplemented with vitamin C alone or combined with other supplements.29 In our trial, women assigned to the vitamin group were less likely to deliver preterm (<37 weeks' gestation) than were women taking placebo. The VIP trial raised concerns about the effect of antioxidant supplementation on growth restriction in a subgroup of women with diabetes.18 Our trial, in a much larger diabetic population than the VIP trial subgroup, did not confirm this finding. By contrast, women in DAPIT assigned to receive vitamins tended to have a reduced risk of having a baby with birthweight lower than the tenth centile for gestational age. Additionally, the small subgroup with diabetes in the VIP trial contained women with either type 1 or type 2 diabetes, whereas DAPIT recruited only women with type 1 diabetes. Although the pathways involved in pathogenesis of pre-eclampsia could differ between women with type 1 and type 2 diabetes, the VIP result was more likely to have been a chance finding, since the diabetes group in this trial was one of nine subgroups analysed.
Women in our study with a low antioxidant status at baseline (plasma ascorbate <10 μmol/L or serum α-tocopherol ≤5 μmol/mmol cholesterol) who were assigned to receive vitamins had a reduced risk of pre-eclampsia compared with similar women assigned to receive placebo. Though small, these subgroups were almost mutually exclusive. No analysis of this type has been reported for previous trials. Previous negative studies are likely to have been done in women with adequate baseline antioxidant status.18 The exception might have been the WHO trial,19 in which the study population was from developing countries and which did not show any beneficial effect of antioxidant supplementation. However, no measurements of ascorbate or α-tocopherol were available, and women were only presumed to have low antioxidant status on the basis of data from previous studies in the participating clinics. Recruitment of a sufficient cohort of women with low baseline antioxidant status to a randomised trial to confirm our finding would be difficult. However, further characterisation of these patients in an effort to identify women potentially at risk of antioxidant depletion in the clinical setting will be of interest.
Finally, how should these findings be interpreted? The initial report of a beneficial effect of antioxidant vitamins17 could have been a chance finding. Subsequent trials recruited women with various risk factors for pre-eclampsia, and the presence of disparate disease processes and thus pathophysiology might have reduced likelihood of identification of a treatment effect. Our study in a homogeneous population of women with type 1 diabetes offers additional insight into disease mechanisms. In principle, the notion that oxidative stress is implicated in pathogenesis of pre-eclampsia remains plausible, but the benefit of vitamin supplementation might be limited to women with vitamin depletion; however, this idea needs confirmation. Dietary intervention rich in various antioxidants might have benefits that cannot be replicated by individual supplements. Alternatively, prescription of antioxidant vitamins at 8–22 weeks' gestation might be too late to affect the pathological process for most patients with diabetes. Antioxidant vitamins reduce rates of fetal malformation in rats with experimentally induced diabetes;30 however, testing of such a hypothesis in patients would necessitate the introduction of supplementation before or around conception in a much larger number of women.
Acknowledgments
Acknowledgments
This study is funded by grants 067028/Z/02/Z and 083145/Z/07/Z from The Wellcome Trust (registered charity number 210183). We thank the Clinical Research Support Centre, The Royal Hospitals, Belfast, UK, and the Centre for Public Health, Queen's University Belfast, UK for their assistance with data entry; Victoria Pharmaceuticals, The Royal Hospitals, Belfast, UK for packaging and distribution of trial drugs; LifeScan Inc for the supply of blood-glucose meters to trial participants; and Action on Pre-eclampsia (registered charity number 1013557) for publicity.
Contributors
DRM, ISY, MJAM, DWMP, and JDW designed and promoted the study. ISY sought ethics approval. VAH coordinated all aspects of the trial, managed the data, and wrote the first draft of the final report. CCP provided statistical advice and analysed the data. All authors helped to prepare the final report.
The DAPIT Study Group
Site research midwives or coordinators and principal investigators (number of patients recruited shown in parentheses): Scotland L McErlean (research midwife, Aberdeen), D W M Pearson (Aberdeen Maternity Hospital; 71), E A Cameron, M H Crombie (research midwives, Dundee), G Leese (Ninewells Hospital; 16), B A Hamilton (research coordinator, Central Scotland), A MacLeod, J D Walker (St John's Hospital at Howden, Livingston; 34), C Alexander, A W Patrick (Royal Infirmary of Edinburgh, Edinburgh; 39), C Love, M W J Strachan (Western General Hospital, Edinburgh; 16), R Lindsay, C B Lunan, F Mackenzie, K Paterson (Princess Royal Maternity Hospital, Glasgow; 55), A D Cameron, M Small (The Queen Mother's Maternity Hospital, Glasgow; 28), A P Gallagher, J Gibson, S Pringle (Southern General Hospital, Glasgow; 31), D McLellan, K McKenna (Wishaw General Hospital, Wishaw; 37); H D MacPherson, P Holmes, L Buchanan, C Kelly (Stirling Royal Infirmary & Falkirk and District Royal Infirmary; 17), P Allcoat (research nurse, Fife), R Urquhart (Forth Park Maternity Hospital; 7); Northwest England S Hooper (research midwife, Birmingham), F Dunne, H Gee (Birmingham Women's Hospital; 32), A Basu (City Hospital), J Milles (Good Hope Hospital; 12), C Balachandar (Walsall Hospital; 18), H Longworth (research midwife, Liverpool), S Walkinshaw (Liverpool Women's Hospital; 40), I Casson (University Hospital Aintree; 17), J Marshall (research midwife, Manchester), M J A Maresh (St Mary's Hospital for Women and Children; 52), R Young (Hope Hospital, Salford; 1); Northern Ireland C Corry, U G Donnelly, O M King, A T Langan, S Loughridge (research midwives), K Moles (Altnagelvin Area Hospital; 41), A Kennedy (Antrim Area Hospital; 9), K Ritchie (Craigavon Area Hospital; 31), D R McCance, S Tharma, A I Traub (Royal-Jubilee Maternity Service, Belfast; 135), R Harper (Ulster Hospital, Dundonald; 23).
Collaborators: M Clark, A Szczepura (economic analysis), C C Patterson (statistical analysis), A Hill (dietician), H Halliday (neonatologist), M McFarland (trial pharmacist).
Clinical coordinating centre: V A Holmes, O M King, S Loughridge, D R McCance, I S Young.
Central laboratory: H Krueger, C Mercer, L McGonigle, G C McKeeman, C McMaster, J V Woodside, I S Young, A McGinty, K Pogue, P Johnston, S E Gilchrist.
Trial steering committee: I Allen (chair), V A Holmes, M J A Maresh, D R McCance, D W M Pearson, J D Walker, I S Young.
Independent data monitoring committee: D Elbourne (chair), R B Fraser, E Hey.
Conflicts of interest
We declare that we have no conflicts of interest.
Web Extra Material
References
- 1.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 2.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:549–550. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villar J, Say L, GuImezoglu AM. Eclampsia and pre-eclampsia: a health problem for 2000 years. In: Critchley H, MacLean A, Poston L, Walker J, editors. Pre-eclampsia. RCOG Press; London, England: 2003. pp. 57–72. [Google Scholar]
- 4.Meis PJ, Goldenberg RL, Mercer BM. The preterm prediction study: risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol. 1998;178:562–567. doi: 10.1016/s0002-9378(98)70439-9. [DOI] [PubMed] [Google Scholar]
- 5.Garner PR, D'Alton ME, Dudley DK, Huard P, Hardie M. Pre-eclampsia in diabetic pregnancies. Am J Obstet Gynecol. 1990;163:505–508. doi: 10.1016/0002-9378(90)91184-e. [DOI] [PubMed] [Google Scholar]
- 6.Jensen DM, Damm P, Moelsted-Pedersen L. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care. 2004;27:2819–2823. doi: 10.2337/diacare.27.12.2819. [DOI] [PubMed] [Google Scholar]
- 7.Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies—a large, population-based study. Diabetes Care. 2009;32:2005–2009. doi: 10.2337/dc09-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, McLaughlin MK. Lipid peroxidation in pregnancy: new perspectives on pre-eclampsia. Am J Obstet Gynecol. 1989;161:1025–1034. doi: 10.1016/0002-9378(89)90778-3. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JM, Hubel CA. The two stage model of pre-eclampsia: variations on the theme. Placenta. 2009;30(suppl A):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet. 1999;354:788–789. doi: 10.1016/S0140-6736(99)80002-6. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez C, Ruiz E, Gussinye M, Carrascosa A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care. 1998;21:1736–1742. doi: 10.2337/diacare.21.10.1736. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Gallan P, Carrascosa A, Gussinye M, Dominguez C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med. 2003;34:1563–1574. doi: 10.1016/s0891-5849(03)00185-0. [DOI] [PubMed] [Google Scholar]
- 13.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 14.Peuchant E, Brun JL, Rigalleau V. Oxidative and antioxidative status in pregnant women with either gestational or type 1 diabetes. Clin Biochem. 2004;37:293–298. doi: 10.1016/j.clinbiochem.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Toescu V, Nuttall SL, Martin U. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin Sci (Lond) 2004;106:93–98. doi: 10.1042/CS20030175. [DOI] [PubMed] [Google Scholar]
- 16.Holmes VA, McCance DR. Could antioxidant supplementation prevent pre-eclampsia? Proc Nutr Soc. 2005;64:491–501. doi: 10.1079/pns2005469. [DOI] [PubMed] [Google Scholar]
- 17.Chappell LC, Seed PT, Briley AL. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354:810–816. doi: 10.1016/S0140-6736(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 18.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH, for the Vitamins in Pre-eclampsia (VIP) Trial Consortium Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 19.Villar J, Purwar M, Merialdi M. World Health Organization multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG. 2009;116:780–788. doi: 10.1111/j.1471-0528.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 20.Holmes VA, Young IS, Maresh MJ. The Diabetes and Pre-eclampsia Intervention Trial. Int J Gynaecol Obstet. 2004;87:66–71. doi: 10.1016/j.ijgo.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Spinnato JA, 2nd, Freire S, Pinto E. Antioxidant therapy to prevent pre-eclampsia: a randomised controlled trial. Obstet Gynecol. 2007;110:1311–1318. doi: 10.1097/01.AOG.0000289576.43441.1f. [DOI] [PubMed] [Google Scholar]
- 22.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS, ACTS Study Group Vitamins C and E and the risks of pre-eclampsia and perinatal complications. N Engl J Med. 2006;354:1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Perez-Cuevas R, Xiong X. An international trial of antioxidants in the prevention of preeclampsia (INTAPP) Am J Obstet Gynecol. 2010;202:239.e1–239.e10. doi: 10.1016/j.ajog.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Roberts JM, Myatt L, Spong CY. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet. 1992;339:283–287. doi: 10.1016/0140-6736(92)91342-6. [DOI] [PubMed] [Google Scholar]
- 26.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–429. [PubMed] [Google Scholar]
- 27.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13:1341–1356. doi: 10.1002/sim.4780131308. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Hopewell S, Schulz KF. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rumbold A, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2005;2 doi: 10.1002/14651858.CD004069.pub2. CD004072. [DOI] [PubMed] [Google Scholar]
- 30.Cederberg J, Eriksson UK. Antioxidative treatment of pregnant diabetic rats diminishes embryonic dysmorphogenesis. Birth Defects Res A Clin Mol Teratol. 2005;73:498–505. doi: 10.1002/bdra.20144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.