FIGURE 5.
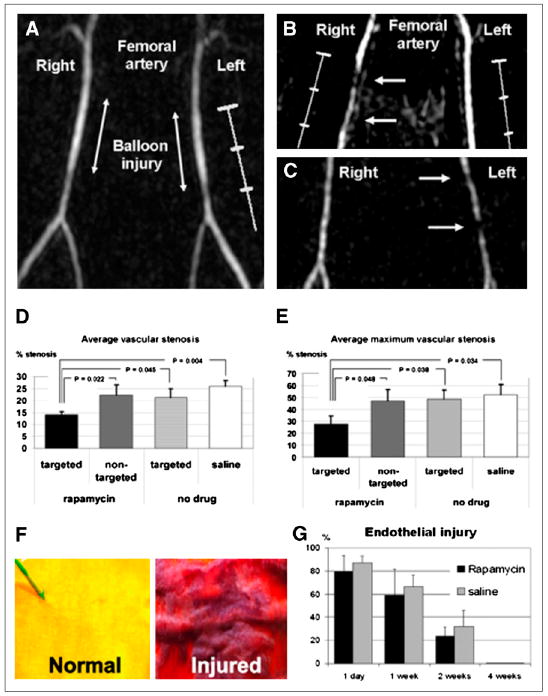
(A) Time-of-flight MR angiogram 30 min after balloon stretch injury shows patent femoral arteries. Left artery was treated with αvβ3-integrin– targeted paramagnetic nanoparticles with rapamycin, and saline was used for right artery. (B and C) MR angiograms 2 wk after injury and treatment, with arrows identifying regions of intraluminal plaque caused by balloon overstretch injury. In B, right artery, which has arterial plaque, was treated with αvβ3-integrin–targeted nanoparticles without drug, and widely patent left artery was treated with αvβ3-integrin– targeted nanoparticles with rapamycin. In C, widely patent right artery was treated with αvβ3-integrin–targeted nanoparticles with rapamycin, and partially occluded left artery was treated with nontargeted nanoparticles with rapamycin. (D and E) Graphs of average (D) and maximum average (E) stenosis within injured and treated femoral arteries of New Zealand White rabbits 2 wk after balloon injury. Arterial segments were flash-frozen in optimal-cutting-temperature compound, and alternate 7-μm sections were used for morphologic analysis (hematoxylin and eosin staining). (F) Area at risk of injured endothelium quantified on vascular en face preparations stained with Carstair stain. Normal, uninjured endothelium is yellow, and injured endothelium with fibrin deposition is red. (G) Quantitation of injured endothelium in area at risk (100% = 1-cm excised vessel segment). Digitized images were analyzed on areas that had undergone balloon overstretch injury and were treated with αvβ3-integrin–targeted nanoparticles with 0.4 mol% rapamycin (n = 12) or saline control (n = 12). Vessels were excised on postinterventional days 1, 7, 14, and 28 (n = 3 per group and time point). (Adapted with permission of (91).)
