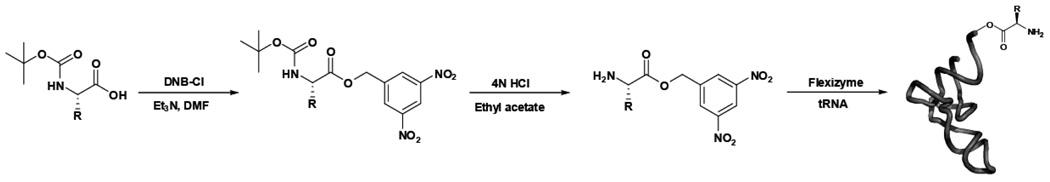
Scheme 1. The aminoacyl-dinitrobenzyl ester substrates for the ribozyme were prepared by chemical synthesis.
Commercial N-tBOC amino acids (or otherwise appropriately protected amino acids) (5, 6) were converted to the dinitrobenzyl esters using dinitrobenzyl chloride. The tBOC protecting group was removed using concentrated hydrochloric acid. The final aminoacyl-dinitrobenzyl esters were recrystallized. tRNAs were then charged with the desired amino acid using the Flexizyme ribozyme. The tRNA and aminoacyl-active ester were incubated with the Flexizyme at high Mg2+ concentration. Charged aa-tRNA was used directly for translation reactions with no further purification. Thus, the Flexizyme ribozyme provided a general, straightforward method for the rapid synthesis of misacylated tRNAs.