Abstract
Asthma, which affects more than 22 million people in the U.S. every year, poses a significant clinical and economic burden to our health care system. Patients, health care practitioners, and payers require a variety of resources to ensure optimal disease management and positive clinical outcomes while also managing costs. In addition, decision makers in health care must determine the most appropriate and cost-efficient therapy or class of agents to achieve asthma control. As such, payers rely on evidence-based medicine, including guidelines to determine the right therapy for the right patient.
Inhaled corticosteroid (ICS) therapy plays a critical role in the management of mild-to-moderate persistent asthma. Despite national treatment guidelines that cite ICS therapy as the most effective and safest long-term treatment option for persistent asthma, ICS monotherapy continues to be underused. One retrospective claims study found that 55.2% of children with mild-to-moderate asthma received prescriptions for combination therapy (ICS and long-acting beta-agonists) as initial controller treatment. This practice is contrary to national treatment guidelines, which recommend a step-therapy approach. These prescribing patterns result in higher pharmacy costs, do not always ensure control of symptoms, and sometimes expose patients to potential safety risks.
This article addresses the importance of ICS therapy in the treatment of mild-to-moderate asthma, as advocated by the National Asthma Education and Prevention Program (NAEPP) Expert Panel Report 3 guidelines; the role of small airway disease in asthma pathophysiology; and the clinical and economic benefits of ICS therapy.
Keywords: asthma, inhaled corticosteroid, disease management, cost effectiveness
INTRODUCTION
Asthma is a prevalent, chronic, disabling condition that can be both challenging to treat and costly to manage.1,2 In recent years, the role of the small airways in asthma pathophysiology has been well documented.3–6
In its updated 2007 guidelines, the National Asthma Education and Prevention Program (NAEPP) reinforced the value of inhaled corticosteroid (ICS) therapy for mild, persistent asthma within all age groups, including children.7 Unfortunately, although ICS therapy is effective and cost-efficient for asthma management, it is often underused by health care professionals, who prescribe either combination therapy or a switch to other agents.8
As the health care arena continues to evolve and as costs continue to rise, decision makers are being constantly challenged to provide quality, cost-efficient care. Because the economic consequences of asthma represent a significant burden to many health plans, payers need to continually evaluate the most up-to-date data to ensure that patients are receiving the best care at the best price.
A variety of programs and initiatives have been undertaken to improve the quality of health care while controlling costs, including disease management, pay for performance, and value-based benefit design. Recently, the federal government has intensified its commitment to lowering costs by including comparative effectiveness research (CER) into the health care system.9 The goal of CER is to give health care decision makers another tool for evaluating treatment options for chronic diseases, such as asthma, which have a large impact on individuals and society.
ECONOMIC BURDEN OF ASTHMA
It is estimated that more than 22 million Americans have asthma, and more than six million of these are children.1 For almost three decades, the prevalence of asthma has been increasing in all sex, age, and racial groups,10 making it one of the most widespread—and costly—diseases in the U.S. today (Table 1).1,2,11 Although these large numbers define the overall burden of a disease, they can seem abstract, making it difficult to comprehend the impact on individuals rather than society as a whole. Every day, as a result of asthma, 40,000 people miss work or school; 5,000 people go to an emergency department (ED) to seek treatment; 1,000 people are admitted to a hospital; and 11 people die—bringing the total annual number of deaths to more than 4,000 as a direct result of asthma.10
Table 1.
Asthma by the Numbers in the U.S.
| Prevalence | |
| Americans affected (2005)1 | |
| Total | ∼22.2 million (7.7%) |
| Children | 6.5 million (8.9%) |
| Health Care Utilization | |
| Office-based physician visits (2007)11 | 10.6 million |
| Emergency department visits (2004)1 | |
| Total | 1.8 million |
| Children | >754,000 |
| Hospitalizations (2004)1 | |
| Total | 497,000 |
| Children | 198,000 |
| Deaths (2006)11 | 3,613 |
| Cost | |
| Costs (2007)2 | |
| Direct | $14.7 billion |
| Indirect | $5.0 billion |
| Missed school days,* children 5 to 17 years of age (2003)1 | $12.8 million |
| Missed work days,* employed adults 18 years of age or older (2003)1 | $10.1 million |
In 2007, the estimated total cost of asthma was almost $20 billion.2 This figure included direct costs (physician visits, hospitalizations, medications) as well as indirect costs (lost workdays and decreased productivity) (see Table 1).1,2,11
Therefore, optimizing disease management of asthma is an ongoing and critical challenge. For insurance companies and other payers, effective management of asthma is important, not only to improve outcomes but also to reduce health care utilization and costs. Patients with uncontrolled asthma have health care costs that are two times higher than those of patients with controlled disease, and costs rise with the increasing severity of asthma.12,13
CLINICAL OVERVIEW OF ASTHMA
Asthma is a common, complex lung disease characterized by recurring and variable symptoms, obstructed airflow, bronchial hyperresponsiveness, and underlying inflammation.5,7 Symptoms can vary greatly within and among individuals.7 Hallmark symptoms are difficulty breathing, coughing (especially at night), tightness, pain or pressure in the chest, and wheezing. Exacerbations can be caused by a multitude of factors, including infections, environmental and occupational allergens, exercise, inhaled irritants, and emotion.14 These episodes can be diverse and can produce symptoms that range in intensity based on disease severity.14
The basic underlying abnormality in asthma is airway inflammation, which has implications for the diagnosis, management, and possibly even the prevention of the disease.7 Historically, inflammation in the central airways has been used to evaluate patients with asthma because (1) the small size of the distal airways (usually 2 mm in diameter or less) makes assessment difficult and (2) the current physiological assessments of lung function are targeted at measuring the function of the large airways.5,6,15
However, inflammation and the resulting airway remodeling that occur in asthma are present throughout the entire airway.16 Research with recent sophisticated technology, such as high-resolution computed tomography (CT), fiberoptic bronchoscopy, and immunohistochemical methods, has validated the concept that disturbances in the smaller airways are indeed integral to the disease process of asthma, including inflammation, airway remodeling, airflow obstruction, airway hyper-sensitivity, nocturnal asthma, severity of disease, and spontaneous exacerbations of symptoms.3–6
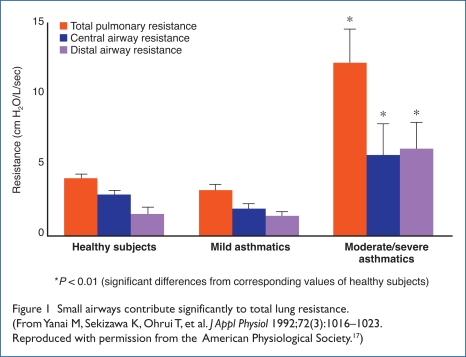
Yanai and colleagues demonstrated that the distal airway contributed dramatically to total lung resistance in patients with moderate-to-severe asthma compared with healthy subjects or those with mild asthma.17 The authors concluded that the peripheral airways are the dominant site of airflow obstruction, regardless of the pathogenesis of the obstruction (Figure 1).17
Figure 1.
Small airways contribute significantly to total lung resistance. (From Yanai M, Sekizawa K, Ohrui T, et al. J Appl Physiol 1992;72(3):1016–1023. Reproduced with permission from the American Physiological Society.17)
In another study, early closure of the small airways was associated with recurrent, severe exacerbations.18 Furthermore, small-airway remodeling is common in fatal asthma, whereas distal lung disease may increase the risk of recurrent asthma exacerbations.5 Because the distal airways (between 0.5 mm and 2 mm in diameter) comprise most respiratory bronchioli,6 they can be a vital target in any disease-management approach for asthma.
Understanding the importance of small-airway disease in asthma pathophysiology is essential for providing optimal treatment; improving outcomes and quality of life; and reducing exacerbations, ED visits, and hospitalizations. With the increasing availability of extra-fine-particle steroids that access the small airways, long-term asthma outcome studies are needed to determine the clinical value of these agents.5,6
BARRIERS TO EFFECTIVE ASTHMA MANAGEMENT
Despite all that is known about asthma, there are still numerous barriers to managing the disease effectively, including underdiagnosis, inaccurate perception of control over the disease, and nonadherence to treatment recommendations and national guidelines.
Asthma in children and adolescents is often underrecognized and underdiagnosed, even though up to 80% of children with asthma experience symptoms before five years of age.7,19,20 Diagnosing asthma in young children is difficult because the symptoms (type, severity, and frequency) vary greatly among these patients. Moreover, asthma in children is often misdiagnosed as chronic bronchitis, reactive airway disease, and recurrent pneumonia, among other conditions.7,20 An awareness of the risk factors for the underdiagnosis of asthma may help the diagnostic process so that more children receive appropriate care at an earlier age.19
People often underestimate how well they are controlling their own or their children’s asthma, a fact that complicates effective management. Children and Asthma in America, a landmark study, was conducted in 2004 to determine the state of asthma and its management among children in the U.S.21 One of the study’s key findings was that parents tended to underestimate the frequency of their child’s symptoms, thereby contributing to an inaccurate perception of control.21 In another study of 207 adults with asthma over a five-year period, patients consistently underestimated the severity of their asthma, even after receiving education and services for long-term medication therapy management.22 The authors concluded that these findings illustrate the importance of the NAEPP’s recommendation for the use of objective measurements in assessing and monitoring asthma control.22
Adherence (or nonadherence) to treatment, or the extent to which a patient’s behavior corresponds with recommendations from a health care professional, can also affect outcomes.23 In asthma, medication adherence rates for inhaled medications vary, but they are often less than 50%.23,24 Exacerbation of disease is often the result of nonadherence, and difficulty controlling asthma should alert practitioners to investigate patient compliance with a pharmacotherapeutic plan.24 Some clinicians take the responsibility of adherence a step further, believing that it is their duty to ensure patient compliance—a commitment that undoubtedly affects outcomes in a positive fashion.24
In addition, the compliance of health care professionals to clinically established guidelines is suboptimal, and this can also contribute to poor outcomes. As demonstrated by the 2004 Children and Asthma in America study, standards for ongoing monitoring of children with asthma did not meet established treatment goals.21 Even though nearly 80% of respondents indicated that their own or their children’s asthma was well or completely controlled, 67% experienced daytime, nighttime, or exercise-induced symptoms; 23% had to make an ED visit; 54% missed school or day care; 42% used quick-relief or rescue medication three times per week to daily; 54% did not have a written asthma action plan; and 25% had not seen their health care provider about their asthma in the previous year.25
A review by Storms cited poor compliance with national guidelines by physicians, pharmacists, and other caregivers as a contributing factor to the undertreatment of asthma.26 The possible reasons for this lack of compliance by physicians are many and varied, including disagreement with the recommendations, either specifically or in general; lack of familiarity, awareness, and training (i.e., of generalists versus specialists); lack of motivation; economic disincentives; and lack of time.23,26
With regard to outcomes, improved adherence to guidelines by health care practitioners may translate into better patient outcomes if the patients, in turn, adhere to their providers’ recommendations.23 The evidence is clear: patients who follow the recommendations of their health care providers tend to do better and have fewer exacerbations, ED visits, and hospitalizations—and, therefore, reduced costs—compared with patients who are nonadherent.22–24,26
Storms also found that certain groups seemed to deviate from the national recommendations, including pediatric, inner-city, and managed care patients.26 In one study of more than 13,000 children with asthma from three managed care organizations (MCOs), there were variations in MCO dispensing patterns of anti-inflammatory agents as well as in the use of health care services.27 These authors concluded that despite guidelines for asthma care, not all children with asthma were receiving appropriate therapy as recommended.27
GUIDELINES FOR TREATMENT
Expert Panel Guidelines
Clinical guidelines are developed when there is enough scientific evidence to warrant a rigorous, systematic review of the published medical literature.28 They offer clinicians strategies on how to best care for their patients, including how to select the appropriate pharmacological therapy based on disease symptoms and severity. Managed care decision makers also use clinical guidelines to help with disease-management approaches and selecting drugs for formularies. In August 2007, the NAEPP issued Expert Panel Report 3 (EPR-3) in its ongoing effort to improve the care of patients with asthma.7 These evidence-based recommendations were derived from the most current data and were issued as an update to selected topics, released in 2002, and to the original guidelines published in 1991 and 1997.28
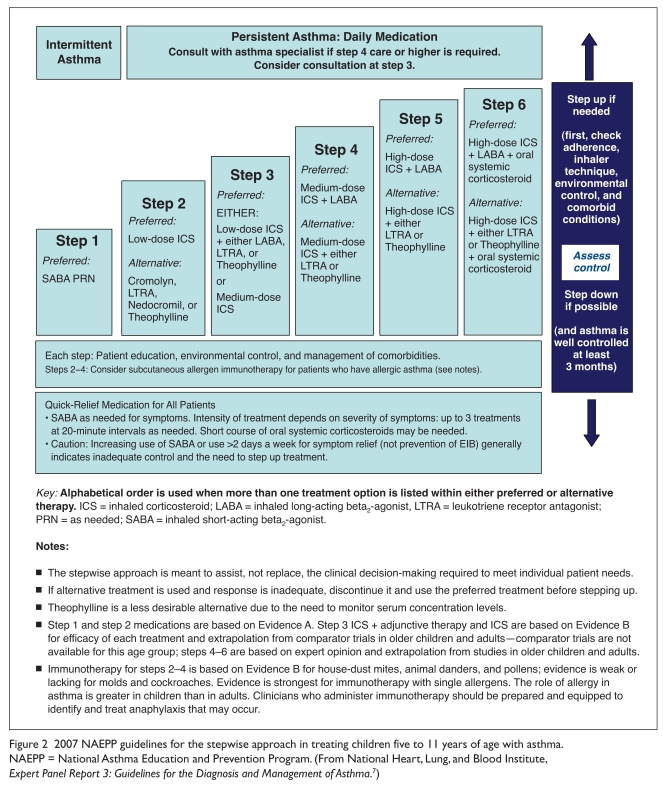
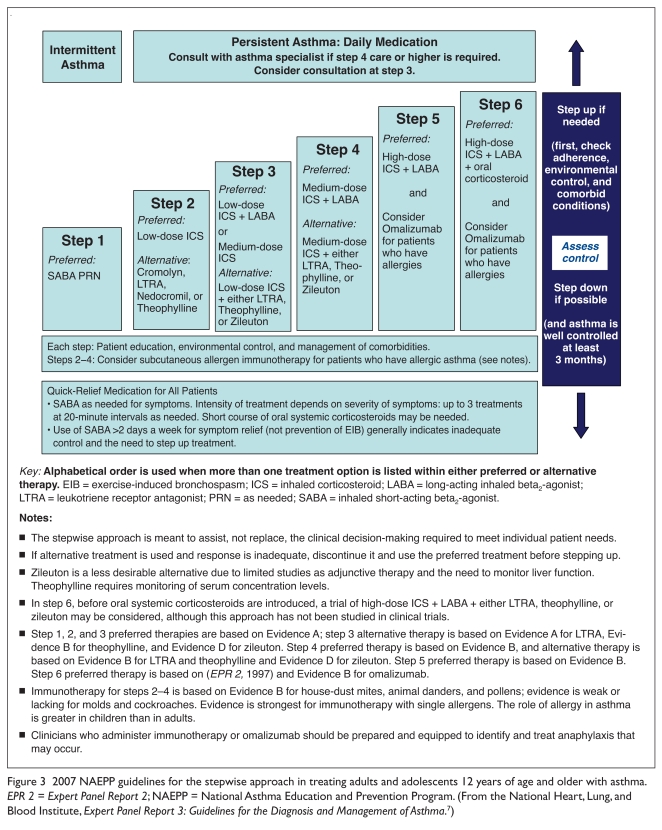
EPR-3 recommend low-dose ICS monotherapy as step-2 therapy for persistent (mild, moderate, or severe) asthma (Table 2) in all age groups, including children (Figures 2 and 3).7 It is well established that ICS agents are considered the most effective daily long-term, anti-inflammatory drugs available for asthma.7 The guidelines also state that for patients whose asthma is not adequately controlled with low-dose ICS monotherapy, clinicians should consider either increasing the dose of the ICS (i.e., medium-dose therapy) or adding a long-acting beta2-agonist (LABA) to the low-dose ICS regimen (step 3).7
Table 2.
Classification of Asthma Severity
| Components of Severity* | Category | ||||||
|---|---|---|---|---|---|---|---|
| Children 5 to 11 Years of Age | Adults and Youths 12 Years of Age or Older | ||||||
| Mild | Moderate | Severe | Mild | Moderate | Severe | ||
| Impairment | Symptoms | More than 2 days per week but not daily | Daily | Throughout the day | More than 2 days per week but not daily | Daily | Throughout the day |
| Nighttime awakenings | 3 to 4 times per month | More than 1 time/week but not nightly | Often 7 times per week | 3 to 4 times per month | More than 1 time per week but not nightly | Often 7 times per week | |
| Short-acting beta2-agonist use for symptom control (not for prevention of exercise-induced bronchospasm) | More than 2 days per week but not daily | Daily | Several times per day | More than 2 days per week but not more than 1 time per day | Daily | Several times per day | |
| Interference with normal activity | Minor limitation | Some limitation | Extremely limited | Minor limitation | Some limitation | Extremely limited | |
| Lung function |
|
|
|
|
|
|
|
| Risk | Exacerbations requiring oral systemic corticosteroids |
|
|
||||
| Lowest level of treatment required to maintain control | Step 2 | Step 3 or 4 | Step 5 or 6 | Step 2 | Step 3 or 4 | Step 5 or 6 | |
FEV1 = forced expiratory volume in one second; FVC = forced vital capacity; ICU = intensive-care unit.
The level of severity is determined by both impairment and risk. The impairment domain is assessed by the patient’s or caregiver’s recall of the previous two to four weeks and spirometry. Severity is assigned to the most severe category in which any feature occurs.
At present, the data are inadequate to correspond frequencies of exacerbations with different levels of asthma severity. In general, more frequent and intense exacerbations (e.g., requiring urgent, unscheduled care, hospitalization, or ICU admission) indicate greater underlying disease severity. For treatment purposes, patients who had two or fewer exacerbations requiring oral systemic corticosteroids in the past year may be considered the same as patients who have persistent asthma, even in the absence of impairment levels consistent with persistent asthma.
From the National Heart, Lung, and Blood Institute. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma.7
Figure 2.
2007 NAEPP guidelines for the stepwise approach in treating children five to 11 years of age with asthma. NAEPP = National Asthma Education and Prevention Program. (From National Heart, Lung, and Blood Institute, Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma.7)
Figure 3.
2007 NAEPP guidelines for the stepwise approach in treating adults and adolescents 12 years of age and older with asthma. EPR 2 = Expert Panel Report 2; NAEPP = National Asthma Education and Prevention Program. (From the National Heart, Lung, and Blood Institute, Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma.7)
Although the EPR-3 guidelines do not recommend combination therapy (ICS plus LABA) for mild, persistent asthma—ICS monotherapy is clearly stated as the treatment of choice—it is generally recognized that clinicians are not following this recommendation and are instead either using a leukotriene modifier (LM) or initiating combination therapy before the full potential of ICS monotherapy is reached.7,8 In a retrospective claims study, Friedman and associates found that despite guideline recommendations, 55.2% of children with mild-to-moderate asthma received the combination of an ICS plus a LABA as initial controller therapy instead of ICS monotherapy.29
NICE Guidelines: Comparative Effectiveness of Inhaled Corticosteroid Therapies
The National Institute for Health and Clinical Excellence (NICE) in the United Kingdom has been issuing CER recommendations for drugs, medical devices, and diagnostic tests since 1999.30 In its technology-appraisal guidance, issued in March 2008, the institute recommended a stepwise approach to the treatment of asthma that was similar to that of the NAEPP.31 Based on the British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN), the NICE committee recommended ICS treatment as step 2 when a person (1) has had asthma exacerbations in the previous two years, (2) is using inhaled short-acting beta2-agonists (SABAs) three times per week or more, (3) is symptomatic three times a week or more, or (4) is awakened at night at least once weekly because of symptoms.31 For patients whose asthma remains uncontrolled, the NICE guidelines recommend either an add-on therapy to the existing ICS therapy or increasing the ICS dose (step 3).31
In its review of ICS therapies, NICE analyzed seven available treatments (five monotherapies and two combination therapies).31 In terms of efficacy, NICE concluded that all ICS therapies were similar in improving symptoms at both low and high doses.31 In its cost-effectiveness analysis of ICS therapies, the NICE committee found that regardless of the dosing range, hydrofluoroalkane (HFA)-propelled beclomethasone dipropionate (BDP) was the least expensive option.31 When non-chlorofluorocarbon (non-CFC) beclomethasone products were excluded from the assessment, the group found that BDP HFA (e.g., QVAR, Teva/Ivax) still had the lowest average cost for all dosages.31 *
After its thorough and clinical cost-effectiveness evaluations of ICS therapies, the NICE committee, keeping the BTS/SIGN asthma-management guidelines in mind, concluded that BDP HFA, with its delivery system consisting of smaller ICS particles, led to improved lung deposition.31 The NICE guidelines embraced the fundamental nature of comparative effectiveness, restating that the least costly product that is appropriate (within its approved parameters) for an individual is recommended.31,32
BENEFITS OF INHALED CORTICOSTEROIDS
Health care decision makers are faced with the task of choosing the most appropriate therapy for a patient, which often involves balancing the value of the treatment, both clinically and economically. As an initial treatment option, ICS therapy is considered to be a clinically viable choice for mild-to-moderate asthma, but it is also a choice with economical value.
Two large retrospective analyses evaluated the costs and resource utilization of patients with asthma.8,33
Colice et al. reviewed data from a large privately insured claims database from 31 companies in the U.S. from 1999 through 2005.8 Of 1,283 patients with mild asthma who met the entry criteria, 319 initiated regular ICS use, 414 initiated ICS plus LABA therapy, and 550 initiated LM treatment.8 The analysis showed that physicians were not following recommended guidelines; instead, they were more likely to prescribe LMs or ICS agents plus a LABA for mild, persistent asthma.8 The authors concluded that although outcomes were similar for all three groups—including asthma-related hospitalizations, ED visits, and SABA use—treatment with ICS monotherapy was less expensive, with lower asthma-related direct costs.8
Zeiger et al. also conducted a large retrospective analysis of the costs and resource utilization of patients with asthma using data from the extensive database of Kaiser Permanente Southern California health plan (N = 96,631), collected from 2002 to 2004.33 Adjusted total and asthma-related drug costs were lower with the use of ICS monotherapy than with most other monotherapies and almost all combination regimens.33 Furthermore, asthma-related resource utilization (i.e., hospitalization or ED visits related to asthma or the use of oral corticosteroids) was also lower for ICS monotherapy compared with LMs and most combination therapies.33
The results from these two studies8,33 were similar to those from a large observational study by Thomas and colleagues.34 The Thomas study showed that increasing the ICS dose could result in a lower risk of severe exacerbations and hospitalizations compared with adding a LABA.34 In an analysis of more than 64,000 patients, the authors found the odds for successful, versus partially successful or unsuccessful, treatment were similar for patients receiving either stepped-up ICS therapy or ICS/LABA therapy (odds ratio[OR], 1.00).34 However, compared with the ICS/LABA group, a higher proportion of patients in the ICS group had successful and partially successful outcomes, compared with unsuccessful treatment (OR, 1.22).34 Patients who received optimized ICS treatment also had a 31% lower risk of hospitalization resulting from respirator y problems, compared with patients receiving an ICS/LABA combination.34
ICS agents, despite their actions via similar mechanisms, have variations in safety and efficacy that may be related to their differences in their particle size and delivery (Table 3).35–37 In a 2009 review, Baptist and Reddy concluded that ICS therapies could differ in both safety and effectiveness for individual patients.38 For example, in a 2009 evaluation performed by Berger et al., ciclesonide (e.g., Alvesco, Sepracor) improved FEV1 and morning peak expiratory flow from baseline in subjects 12 years of age and older who were not then using an ICS therapy.39 However, the overall incidence of adverse events was similar between the treatment groups (range, 53%–58%).39
Table 3.
Particle Size and Differences in Delivery among Inhaled Corticosteroid Therapies
| Drug | Formulation | Particle Size (microns) | Lung Deposition |
|---|---|---|---|
| Fluticasone DPI (Flovent Diskus, GlaxoSmithKline)5 | Dry powder | 5.4 μm | 15% |
| Triamcinolone CFC* (Azmacort, Abbott)5,35 | Suspension | 4.5 μm | 22% |
| Flunisolide CFC* (Aerobid Inhaler System, Forest)35 | Suspension | 3.8 μm | 20% |
| BDP CFC (Vanceril, Schering-Plough)35 | Suspension | 3.5 μm | 4% |
| Fluticasone CFC (Flovent, CFC Inhalation Aerosol, GlaxoSmithKline)5,35 | Suspension | 2.4 μm | 26% |
| Fluticasone HFA (Flovent HFA Inhalation Aerosol, GlaxoSmithKline)35 | Suspension | 2.4 μm | Unknown |
| BDP HFA (QVAR HFA Inhalation Aerosol,Teva)35,36 | Solution | 1.1 μm | >56% |
| Ciclesonide HFA (Alvesco, Sepracor)37 | Solution | 1–2 μm | 52% |
| Flunisolide HFA (Aerospan, Forest)35 | Solution | 1.2 μm | 68% |
BDP = beclomethasone dipropionate; CFC = chlorofluorocarbon; DPI = dry powder inhaler; HFA = hydrofluoroalkane; ICS = inhaled corticosteroid.
In April 2010, the FDA ordered inhalers containing CFC propellants (including Aerobid and Azmacort) to be gradually removed from the market in the U.S., because CFCs deplete the ozone layer.
Corren and associates established that flunisolide HFA (Aerospan, Forest), 170 mcg twice daily and 340 mcg twice daily, showed significant improvement in the percentage of increase in FEV1 (12.22% and 14.69%, respectively; P < 0.01) at one-third the dose of flunisolide CFC (Aerobid, Forest), compared with the placebo group (5.35%).40 In their 2007 evidence-based review of ICS therapies, Abdullah and Khan concluded that although flunisolide caused fewer side effects than other ICS agents, it was also relatively less effective.41
Traditionally, ICS products are aerosol formulations that contain suspended solid drug particles delivered by a propellant. This type of formulation and delivery results in larger particle sizes (2.4–4.5 microns), which are deposited primarily in the central airways, resulting in low total lung deposition.5,42–44 Many standard ICS treatments also rely on a spacer for improved drug delivery. Unfortunately, many patients do not use the proper technique when administering their ICS therapy. The combination of larger particle sizes and poor inhaler technique often results in up to 90% of the drug being deposited in the mouth and pharynx, sometimes leading to adverse events such as oral candidiasis.36,38
Unlike traditional ICS therapies, BDP HFA involves a liquid solution; a spacer is not required. The small particle size of BDP HFA (1.1 microns)15,35,36 potentially enables more of the drug to reach both the large and small airways, which may result in comprehensive lung and peripheral lung deposition.44 Both the NAEPP EPR-3 and NICE guidelines note that a small particle size enables the delivery of more drug to the lung than other ICS therapies with larger particles.7,31 The ability to reach the distal lungs and treat chronic small-airway inflammation potentially provides more successful asthma control, possibly leading to fewer exacerbations and a subsequent reduction in the use of health care resources.45 Table 3 illustrates the variation in particle size and lung deposition rates for various ICS therapies.5,35–37
Kemp and coworkers reported on the real-life effectiveness of BDP HFA therapy in patients identified in the General Practice Research Database, a large, representative clinical database in the United Kingdom.45 They compared the efficacy of BDP HFA monotherapy (QVAR) with BDP CFC (e.g., Vanceril, Schering-Plough) and fluticasone propionate HFA and CFC (Flovent, GlaxoSmithKline) metered-dose inhalers.45 Patients (N = 4,133) were five to 60 years of age, and they had no other chronic respiratory diseases. At the 12-month assessment, BDP HFA was noted to be more likely to provide control of asthma and less likely to result in exacerbations. These findings can be extrapolated to a reduced health care resource utilization and, therefore, cost savings.45
BDP HFA was also associated with lower medical and asthma-related costs when compared directly with fluticasone, another ICS. Lage and colleagues examined outcomes data from MedStat MarketScan Commercial Claims and Encounters database for 13,968 individuals from July 1, 2002, through June 30, 2007.46 BDP HFA was administered to 3,223 patients, and 10,745 patients received fluticasone. Total direct medical costs were significantly lower with BDP HFA ($5,063) than with fluticasone ($5,377) (P = 0.0042).46
These results are likely driven by the significantly lower drug costs for BDP HFA compared with fluticasone ($2,366 vs. $2,581, respectively; P < 0.0001) and significantly lower ED costs ($185 vs. $249, respectively; P < 0.0001). BDP HFA was also associated with significantly lower asthma-related out-patient costs, compared with fluticasone ($191 vs. $224, respectively; P < 0.0001) and asthma-related ED costs ($28 vs. $45, respectively; P < 0.0001).46
Patients receiving BDP HFA also had a lower risk for an ED visit from any cause (a 17% reduction) and a lower risk for asthma-related ED visits (a 30% reduction) during the one-year follow-up period.46
CONCLUSION
As a result of the significant clinical and economic burden associated with uncontrolled asthma, improved therapeutic outcomes for these patients are crucial for lowering the use of health care resources and costs. However, numerous barriers prevent the optimal control of asthma, including the inappropriate use of combination therapy as the initial treatment approach. MCOs should work with clinicians to ensure a better understanding about the importance of following validated asthma guidelines for their patients with mild-to-moderate, persistent asthma, including an emphasis on the need to optimize ICS monotherapy instead of switching to another agent or implementing combination therapy. The evidence indicates that optimizing ICS use not only improves patient outcomes but also is a safe, cost-effective approach.
BDP HFA, a small-particle ICS, reaches the distal lungs, resulting in comprehensive and peripheral lung deposition. Based on a 2008 analysis by NICE, the unweighted mean cost of BDP HFA was found to be the least expensive option compared with other available ICS therapies, and subsequent studies have found it to be more cost-effective than fluticasone.31
Both the NAEPP EPR-3 and NICE guidelines advocate a step-care approach to asthma therapy and recommend ICS agents as the preferred therapy for mild-to-moderate, persistent asthma. Despite national guidelines, however, clinicians often use combination therapy as an initial treatment strategy. MCOs should emphasize the clinical benefits of ICS monotherapy to health care practitioners, as advocated by the NAEPP EPR-3 guidelines. Among the available ICS therapies, BDP HFA has been shown to be an effective, cost-efficient option.
Acknowledgments
The author thanks Kathlene Graziano from Strategic Healthcare Alliance and Deborah L. Ross, medical writer, for their assistance in preparing this manuscript.
Footnotes
In April 2010, the FDA ordered inhalers containing CFC propellants to be gradually removed from the market in the U.S. (e.g., Aerobid and Azmacort) because CFCs deplete the ozone layer.
Disclosure: This work was funded by Teva Pharmaceuticals USA, marketer of beclomethasone dipropionate hydrofluoroalkane inhalation aerosol (QVAR). Dr. Beam reports that he has no direct relationship, financial or otherwise, with Teva Pharmaceuticals USA.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) Asthma Prevalence, Health Care Use and Mortality: United States, 2003–05 Reviewed November 18, 2009. Available at: www.cdc.gov/nchs/data/hestat/asthma/asthma.htm Accessed June 9, 2010. [Google Scholar]
- 2.American Lung Association Trends in Asthma Morbidity and Mortality Epidemiology and Statistics Unit, Research and Program Services Division. January 2009. Available at: www.lungusa.org/lung-disease/asthma June 9, 2010.
- 3.Kraft M. Location of asthma inflammation and the distal airways: Clinical implications. Part III. Curr Med Res Opin. 2007;23(Suppl 3):S21–S27. doi: 10.1185/030079907X226177. [DOI] [PubMed] [Google Scholar]
- 4.Ueda T, Niimi A, Matsumoto H, et al. Role of small airways in asthma: Investigation using high-resolution computed tomography. J Allergy Clin Immunol. 2006;118(5):1019–1025. doi: 10.1016/j.jaci.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Martin RJ. Therapeutic significance of distal airway inflammation in asthma (review) J Allergy Clin Immunol. 2002;109(2 Suppl):S447–S460. doi: 10.1067/mai.2002.121409. [DOI] [PubMed] [Google Scholar]
- 6.Tashkin DP. The role of small airway inflammation in asthma (review) Allergy Asthma Proc. 2002;23(4):233–242. [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma, Full Report 2007 National Asthma Education and Prevention Program. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf Accessed November 17, 2009.
- 8.Colice GL, Yu AP, Ivanova JI, et al. Costs and resource use of mild persistent asthma patients initiated on controller therapy. J Asthma. 2008;45(4):293–299. doi: 10.1080/02770900801911178. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Congress Research on the Comparative Effectiveness of Medical Treatments: Issues and Options for an Expanded Federal Role Washington, D.C.Congressional Budget Office; 2007. Available at: www.cbo.gov/ftpdocs/88xx/doc8891/12-18-ComparativeEffectiveness.pdf Accessed November 17, 2009. [Google Scholar]
- 10.Asthma and Allergy Foundation of America Asthma Facts and FiguresAvailable at: www.aafa.org/display.cfm?id=8&sub=42 Accessed November 17, 2009.
- 11.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) FastStats for asthma. Updated May 15, 2009. Available at: www.cdc.gov/nchs/fastats/asthma.htm Accessed November 18, 2009.
- 12.Sullivan SD, Rasouliyan L, Russo PA, et al. for the TENOR Study Group Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy. 2007;62(2):126–133. doi: 10.1111/j.1398-9995.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 13.Colice G, Wu EQ, Birnbaum H, et al. Healthcare and work loss costs associated with patients with persistent asthma in a privately insured population. J Occup Environ Med. 2006;48(8):794–802. doi: 10.1097/01.jom.0000229819.26852.0e. [DOI] [PubMed] [Google Scholar]
- 14.Asthma Merck Manuals, online medical library. Available at: www.merck.com/mmpe/sec05/ch048/ch048a.html Accessed November 17, 2009.
- 15.Gross GN. Evaluating and treating small airway disease in asthma. Business Briefing: US Respiratory Care. 2005:1–4. [Google Scholar]
- 16.Vanden Burgt JA, Busse WW, Martin RJ, et al. Efficacy and safety overview of a new inhaled corticosteroid, QVAR (hydrofluoro-alkane–beclomethasone extra fine inhalation aerosol), in asthma. J Allergy Clin Immunol. 2000;106(6):1209–1226. doi: 10.1067/mai.2000.111582. [DOI] [PubMed] [Google Scholar]
- 17.Yanai M, Sekizawa K, Ohrui T, et al. Site of airway obstruction in pulmonary disease: Direct measurement of intrabronchial pressure. J Appl Physiol. 1992;72(3):1016–1023. doi: 10.1152/jappl.1992.72.3.1016. [DOI] [PubMed] [Google Scholar]
- 18.In’t Veen JCCM, Beekman AJ, Bel EH, Sterk PJ. Recurrent exacerbations in severe asthma are associated with enhanced airway closure during stable episodes. Am J Respir Crit Care Med. 2000;161(6):1902–1906. doi: 10.1164/ajrccm.161.6.9906075. [DOI] [PubMed] [Google Scholar]
- 19.Siersted HC, Boldsen J, Hansen HS, et al. Population based study of risk factors for underdiagnosis of asthma in adolescence: Odense schoolchild study BMJ 19983167132651–655.commentaries, 655–657. [PMC free article] [PubMed] [Google Scholar]
- 20.Eigen H. Differential diagnosis and treatment of wheezing and asthma in young children. Clin Pediatr (Phila) 2008;47(8):735–743. doi: 10.1177/0009922808315662. [DOI] [PubMed] [Google Scholar]
- 21.Children and Asthma in America, Executive Summary Overview Available at: www.asthmainamerica.com/children_index.html Accessed November 18, 2009.
- 22.Bunting BA, Cranor CW. The Asheville Project: Long-term clinical, humanistic, and economic outcomes of a community-based medication therapy management program for asthma. J Am Pharm Assoc (2003) 2006;46(2):133–147. doi: 10.1331/154434506776180658. [DOI] [PubMed] [Google Scholar]
- 23.Gillissen A. Patients’ adherence in asthma (review) J Physiol Pharmacol. 2007;58(Suppl 5; Part 1):205–222. [PubMed] [Google Scholar]
- 24.Milgrom H, Bender B, Ackerson L, et al. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98(6, Part 1):1051–1057. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 25.Children and Asthma in America, Executive Summary Missing the Mark Available at: www.asthmainamerica.com/children_missing.html Accessed November 18, 2009.
- 26.Storms WW. Unmet needs in the treatment of allergic asthma: Potential role of novel biologic therapies (review) J Manag Care Pharm. 2003;9(6):534–543. doi: 10.18553/jmcp.2003.9.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donahue JG, Fuhlbrigge AL, Finkelstein JA, et al. for the Pediatric Asthma Care Patient Outcomes Research Team Asthma pharmacotherapy and utilization by children in 3 managed care organizations. J Allergy Clin Immunol. 2000;106(6):1108–1114. doi: 10.1067/mai.2000.111432. [DOI] [PubMed] [Google Scholar]
- 28.National Heart, Lung, and Blood Institute, National Independent Pharmacy Coalition National Asthma Guidelines Updated Bethesda, Md: August292007. Available at: www.nipcweb.com/National_Asthma_Guidelines_Updated.pdf Accessed November 18, 2009. [Google Scholar]
- 29.Friedman HS, Eid NS, Crespi S, et al. Retrospective claims study of fluticasone propionate/salmeterol fixed-dose combination use as initial asthma controller therapy in children despite guideline recommendations. Clin Ther. 2009;31(5):1056–1063. doi: 10.1016/j.clinthera.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Chalkidou K, Tunis S, Lopert R, et al. Comparative effectiveness research and evidence-based health policy: Experience from four countries. Milbank Q. 2009;87(2):339–367. doi: 10.1111/j.1468-0009.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute for Health and Clinical Excellence (NICE) Inhaled Corticosteroids for the Treatment of Chronic Asthma in Adults and in Children Aged 12 Years and OverNICE Technology Appraisal Guidance 138. March 2008. Available at: www.nice.org.uk/nicemedia/pdf/TA138.pdf Accessed November 18, 2009.
- 32.National Institute for Health and Clinical Excellence (NICE) Inhaled Corticosteroids for the Treatment of Chronic Asthma in Adults and in Children Aged 12 Years and Over Quick reference guide. March2008. Available at: www.nice.org.uk/nicemedia/pdf/TA138QuickRefGuide.pdf Accessed November 18, 2009.
- 33.Zeiger RS, Hay JW, Contreras R, et al. Asthma costs and utilization in a managed care organization. J Allergy Clin Immunol. 2008;121(4):885–892. doi: 10.1016/j.jaci.2007.12.1178. [DOI] [PubMed] [Google Scholar]
- 34.Thomas M, von Ziegenweidt J, Lee AJ, Price D. High-dose inhaled corticosteroids versus add-on long-acting beta-agonists in asthma: An observational study. J Allergy Clin Immunol. 2009;123(1):116–121. doi: 10.1016/j.jaci.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 35.Zeidler M, Corren J. Hydrofluoroalkane formulations of inhaled corticosteroids for the treatment of asthma. Treat Respir Med. 2004;3(1):35–44. doi: 10.2165/00151829-200403010-00005. [DOI] [PubMed] [Google Scholar]
- 36.Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ. Influence of particle size and patient dosing technique on lung deposition of HFA–beclomethasone from a metered dose inhaler. J Aerosol Med. 2005;18(4):379–385. doi: 10.1089/jam.2005.18.379. [DOI] [PubMed] [Google Scholar]
- 37.Leach CL, Bethke TD, Boudreau RJ, et al. Two-dimensional and three-dimensional imaging show ciclesonide has high lung deposition and peripheral distribution: A nonrandomized study in healthy volunteers. J Aerosol Med. 2006;19(2):117–126. doi: 10.1089/jam.2006.19.117. [DOI] [PubMed] [Google Scholar]
- 38.Baptist AP, Reddy RC. Inhaled corticosteroids for asthma: Are they all the same? (review) J Clin Pharm Ther. 2009;34(1):1–12. doi: 10.1111/j.1365-2710.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- 39.Berger WE, Kerwin E, Berstein DI, et al. Efficacy and safety evaluation of ciclesonide in subjects with mild-to-moderate asthma not currently using inhaled corticosteroids. Allergy Asthma Proc. 2009;30(3):304–314. doi: 10.2500/aap.2009.30.3242. [DOI] [PubMed] [Google Scholar]
- 40.Corren J, Nelson H, Greos LS, et al. Effective control of asthma with hydrofluoroalkane flunisolide delivered as an extra-fine aerosol in asthma patients. Ann Allergy Asthma Immunol. 2001;87:405–411. doi: 10.1016/S1081-1206(10)62922-5. [DOI] [PubMed] [Google Scholar]
- 41.Abdullah AK, Khan S. Evidence-based selection of inhaled corticosteroid for treatment of chronic asthma. J Asthma. 2007;44(1):1–12. doi: 10.1080/02770900601118099. [DOI] [PubMed] [Google Scholar]
- 42.Cripps A, Riebe M, Schulze M, Woodhouse R. Pharmaceutical transition to non-CFC pressurized metered dose inhalers. Respir Med. 2000;94(Suppl B):S3–S9. [PubMed] [Google Scholar]
- 43.Newman S, Salmon A, Nave R, Drollmann A. High lung deposition of 99mTc-labeled ciclesonide administered via HFA–MDI to patients with asthma. Respir Med. 2006;100(3):375–384. doi: 10.1016/j.rmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 44.Colice GL. Small airway disease: A riddle wrapped in a mystery inside an enigma (editorial) J Allergy Clin Immunol. 2006;118(2):337–339. doi: 10.1016/j.jaci.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Kemp L, Price D, Sims E, et al. ‘Real-life’ effectiveness of QVAR, beclomethasone and fluticasone. J Allergy Clin Immunol. 2009;123(3):729. [Google Scholar]
- 46.Lage MJ, Gross GN, Brewster C, Spalitto A. Outcomes and costs of patients with persistent asthma treated with beclomethasone dipropionate hydrofluoroalkane or fluticasone propionate. Adv Ther. 2009;26(8):762–775. doi: 10.1007/s12325-009-0056-z. [DOI] [PubMed] [Google Scholar]