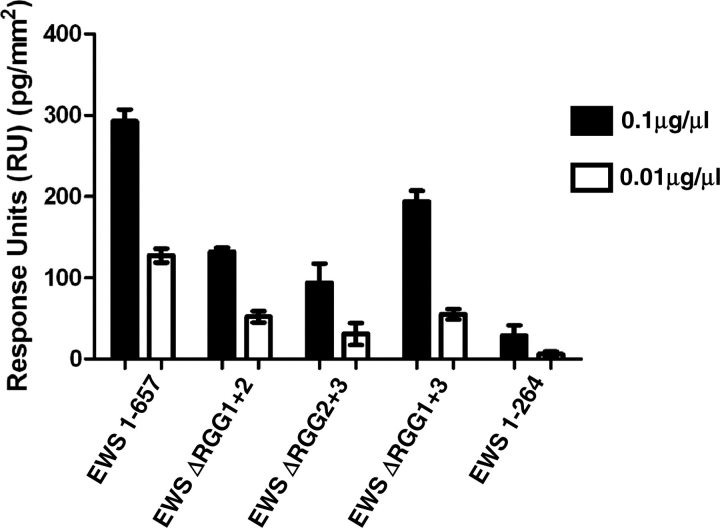
Fig. 4.
Removal of RGG domains blocks the formation of EWS heterodimers. BA-EWS was immobilized on a SA-Biacore chip, and pulsed with: (i) full-length EWS (EWS 1–657; containing RGG1, 2 and 3); (ii) EWS 1–264 (containing no RGG domains); (iii) EWS lacking the first and second RGG-domains (EWS delta RGG1+2, which contains RGG3); (iv) EWS lacking the second and third RGG domains (EWS delta RGG2+3, which contains RGG1); and (v) EWS lacking the first and third RGG domains (EWS delta RGG1+3, which contains RGG2). Protein stocks were diluted to 0.1 and 0.01 µg/µl, and 5 µl of each were injected onto the chip. Injections were repeated three times and the presented figure is the mean value. For all experiments protein binding is represented as RU, with one RU representing 1 pg/mm2 of protein binding to the chip surface, and the values have been standardized for size, using the smallest fragment (1–264), as a reference. Each presented value is the mean of three repeats. At a protein concentration of 0.1 µg/µl EWS 1–657, EWS ΔRGG1+2, and EWS ΔRGG2+3 and EWS ΔRGG1+3 all bind significantly greater than EWS 1–264 with a P < 0.001.