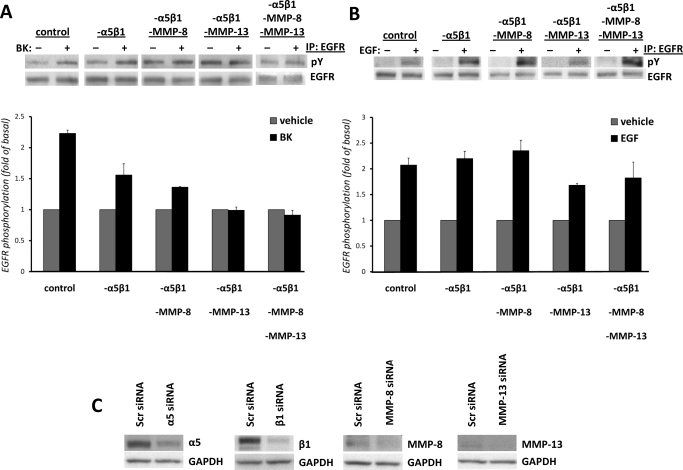
Fig. 4.
Transfection of mIMCD-3 cells with integrin α5β1 and MMP siRNAs decreases BK-induced EGFR phosphorylation. Cells were nucleofected with 100 nM α5β1 siRNA (-α5β1) or -α5β1 with a combination of either MMP-8 siRNA (-α5β1-MMP-8) or MMP-13 siRNA (-α5β1-MMP-13); with a combination of all siRNAs (-α5β1- MMP-8-MMP-13); or with the same amount of control siRNA (control), as described under Materials and Methods. Forty-eight hours after nucleofection, cells were stimulated with vehicle or 100 nM BK (A) or with 1 ng/ml EGF (B) for 5 min, lysed, and analyzed for EGFR phosphorylation as described under Materials and Methods. Experiments were performed at least three times. Data are presented as mean + S.E.M. **, p < 0.01 compared with control BK-treated cells. ANOVA (-α5β1) compared with -α5β1-MMP-8, -α5β1-MMP-13, or -α5β1- MMP-8-MMP-13, not significant. C, Western blot analyses of lysates of mIMCD-3 cells transfected with either scrambled siRNA or siRNAs for α5, β1, MMP-8, and MMP-13 (40 μg of total protein) were performed with commercially available antibodies against α5 and β1 integrin subunits, MMP-8 and MMP-13 to demonstrate down-regulation of these proteins. Blots were stripped and re-probed with antibody against GAPDH to control for the specificity of silencing and protein loading.