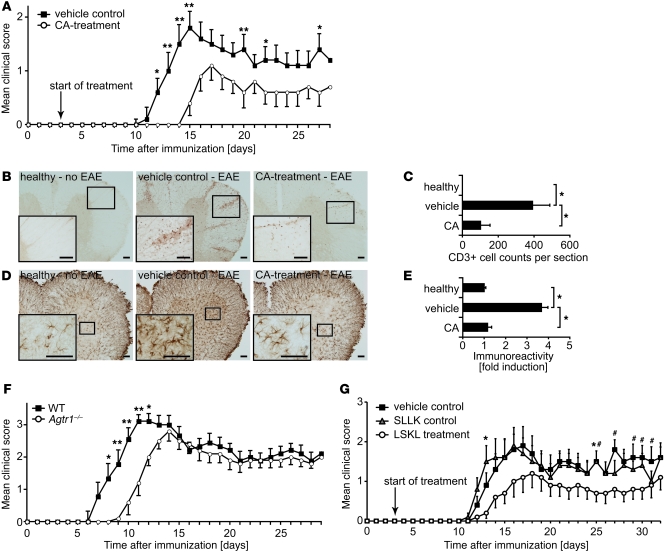
Figure 6. CA treatment delays and attenuates EAE.
(A) Clinical EAE scores comparing vehicle controls with CA-treated mice. CA treatment delays and ameliorates the disease. One representative experiment of 3 independent experiments is shown (n = 10 females per group; mean ± SEM). *P < 0.05, ** P < 0.02 (Mann-Whitney U test). (B) Spinal cord slices stained for CD3. CA treatment suppresses T cell infiltration into the CNS. (C) Statistical evaluation of the experiment shown in B. Count of CD3+ cells per slide. (D) Spinal cord slices stained for GFAP. CA treatment suppresses glial activation. (E) Statistical evaluation of the experiment shown in D. GFAP staining intensities were evaluated with MetaMorph software and normalized on the healthy control group. (B and D) One representative slide of each group is shown. Higher magnification images of the regions in the small boxes are shown in the insets. Scale bars: 50 μm. (C and E) Statistical analyses of 5 spinal cord slices per mouse are shown (n = 5 females per group; mean ± SEM). *P < 0.001 (Student’s t test). (F) Clinical EAE scores comparing WT C57Bl/6 to Agtr1–/– mice. Onset of disease is delayed in Agtr1–/– mice. Results are shown from 2 independent experiments (n = 5 females per group; mean ± SEM). *P < 0.05, **P < 0.02 (Mann-Whitney U test). (G) Clinical EAE scores comparing LSKL treatment with vehicle controls and control peptide–treated (SLLK-treated) mice. Treatment with the TSP-1 inhibitor LSKL ameliorates disease scores (n = 10 females per group; mean ± SEM). *P < 0.05 between LSKL- and SLLK-treated groups, #P < 0.05 between LSKL-treated and vehicle control group (Mann-Whitney U test).