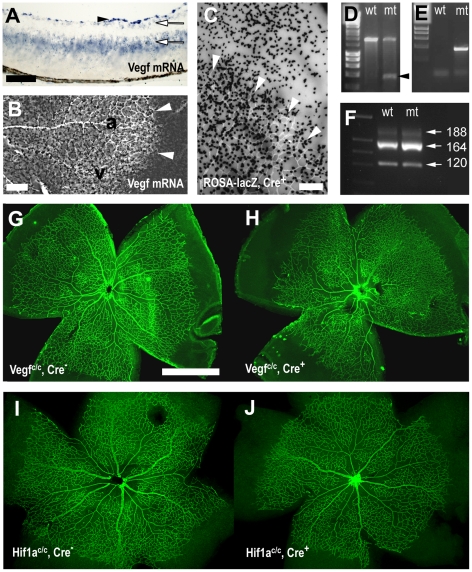
Figure 1. Astrocyte specific deletion of VEGF.
(A, B) In situ hybridisation showed Vegf mRNA expression in a retinal cross-section (A) and a retinal whole mount (B) at P5. (A) Vegf mRNA was most strongly expressed in retinal astrocytes (arrowhead), and weakly in retinal ganglion cells and the inner nuclear layer (arrows). (B) Retinal astrocytes expressed VEGF strongest distally to the edge of growing vascular plexus (white arrowheads), at intermediate levels around veins (v) and weakest along arteries (a). (C) Transgenic mice expressing Cre recombinase in retinal astrocytes (Gfap-Cre mice) showed in a lacZ reporter strain recombination activity (black X-gal stain in C) in retinal astrocytes proximally and distally to the growing edge of the vascular plexus (arrowheads in C). (D, E) Cre recombination in Vegf c/c mice, crossed with Gfap-Cre mice, was demonstrated by PCR across the deleted region in genomic DNA from retinas in Cre positive (mt) and Cre negative (wt) animals (D). PCR on tail DNA identified Cre negative and positive animals (E). (F) PCR on cDNA from retinal mRNA showed unchanged VEGF isoform ratios in control and mutant animals. Astrocyte deletion of VEGF led to subtle abnormalities in the P5 retinal vasculature (stained with an anti-claudin 5 antibody G–J) in Cre positive animals (H) compared to wild type litter mates (G). In contrast, deletion of HIF1α in retinal astrocytes caused no such abnormalities (I–J). Scale bars are 100µm in A, 200µm in B, C and 1000µm in G.