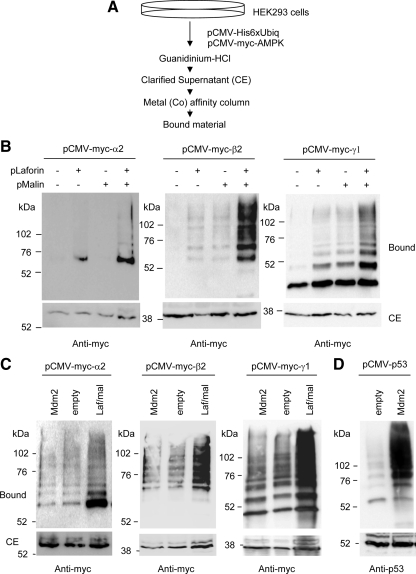
Figure 1.
The laforin–malin complex is able to promote ubiquitination of individual AMPK subunits. (A) Diagram of the protocol used to determine the presence of ubiquitinated proteins, based on the use of a 6xHis-tagged version of ubiquitin (Kaiser and Tagwerker, 2005). (B) HEK293 cells were transfected with plasmid pCMV-His6xUbiq and the indicated combination of plasmids (pLaforin, pCMV-HA-laforin; and pMalin, pcDNA3-HA-malin). Cell extracts were then obtained as described in Materials and Methods and the clarified extract (CE; 40 μg), and the material bound to the metal-affinity chromatography column (bound; 40 μl) was analyzed by SDS-PAGE and Western blotting using anti-myc antibodies. Molecular mass markers are indicated on the left of each panel. For AMPKα2 and AMPKγ1, we also detected a minor modification when only laforin was overexpressed, perhaps because it can force endogenous malin to carry out the corresponding ubiquitination. (C) HEK293 cells were transfected with plasmid pCMV-His6xUbiq and the indicated combination of plasmids (Mdm2, pCMV-Mdm2; Laf/Mal, pCMV-HA-laforin/pcDNA3-HA-malin; and empty, pCMV-HA). Cell extracts were analyzed as described above using anti-myc antibodies. (D) HEK293 cells were transfected with plasmid pCMV-His6xUbiq and plasmids pCMV-p53 and pCMV-Mdm2 or pCMV-HA (empty). Cell extracts were analyzed as described above using anti-p53 antibodies.