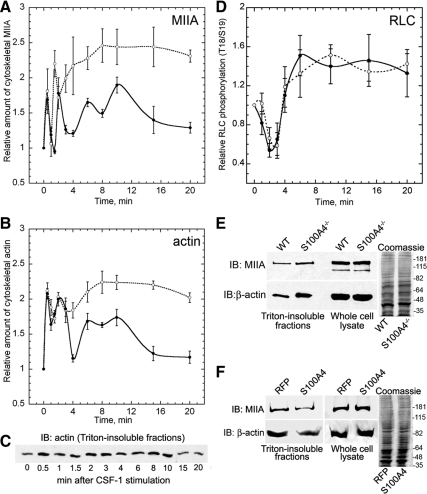
Figure 5.
Loss of S100A4 promotes myosin-IIA and β-actin overassembly but does not affect RLC phosphorylation. Myosin-IIA (A) and β-actin (B) assembly were assessed by isolating Triton X-100–resistant cytoskeletons from CSF-1–stimulated wild-type (solid lines) and S100A4−/− (dotted lines) BMMs. For all curves, values represent the mean ± SEM for three to four independent experiments. (C) Representative immunoblot of β-actin in Triton-insoluble fractions from wild-type BMMs at different times after stimulation with CSF-1. (D) Kinetics of total T18/S19 RLC phosphorylation in stimulated wild-type (solid lines) and S100A4−/− (dotted lines) BMMs. (E) Relative amount of myosin-IIA heavy chain and β-actin in Triton-insoluble fractions and whole cell lysates from wild-type and S100A4−/− BMMs stimulated with CSF-1 for 20 min. Right, Coomassie-stained whole cell lysates used for the immunoblots. (F) Relative amount of myosin-IIA heavy chain and β-actin in Triton-insoluble fractions from S100A4−/− BMMs expressing either TurboRFP or human S100A4 after stimulation with CSF-1 for 20 min. Right, Coomassie-stained whole cell lysates used for the immunoblots.