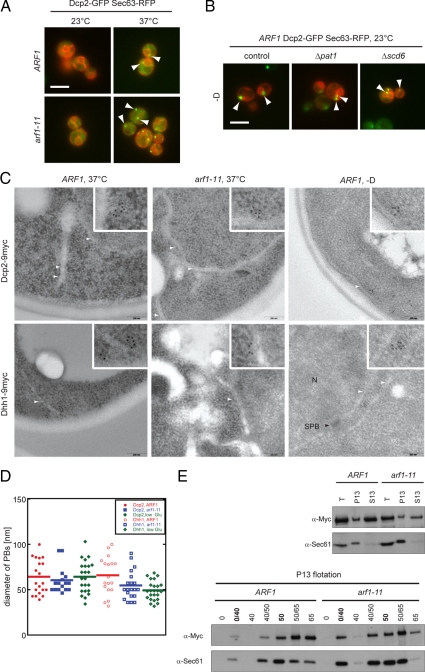
Figure 7.
PBs associate with the ER. (A) Wild-type and arf1-11 mutant cells expressing Dcp2-GFP and the ER marker Sec63p-RFP were shifted to 37°C for 1 h. In both control and arf1-11 cells, PBs were observed next to the ER. White arrowheads point toward a selection of PBs close to the ER. (B) PBs were induced in wild-type cells expressing Dcp2-GFP and the ER marker Sec63p-RFP and deleted for either PAT1 or SCD6 by incubating cells in rich medium without a carbon source for 15 min. In the control as well as in the deletion strains, PBs were observed in proximity to the ER. White arrowheads point toward a selection of PBs close to the ER. White bars (A and B), 5 μm. (C) Dcp2 and Dhh1 are part of spherical structures in proximity to the ER. Wild-type and arf1-11 cells expressing either Dcp2–9myc or Dhh1–9myc were shifted to 37°C for 1 h or incubated in rich medium without a carbon source for 15 min (−D) at 23°C. Cells were fixed and analyzed by immuno-EM for myc. Clusters of gold particles localized next to ER membranes. Scale bar, 200 nm. The inlet is a twofold magnification of the area surrounding PBs. White arrowheads point to ER membranes; N marks a nucleus and SPB with a black arrowhead points to a spindle pole body in the nuclear envelope. (D) Determination of the approximate diameter of PBs. The largest distance between two gold particles in a cluster was determined for Dcp2–9myc and Dhh1–9myc in wild-type and arf1-11 cells. The size of the clusters did not change significantly between different conditions for the two different markers. Each dot represents an individual PB, the horizontal line marks the average. (E) PBs are physically connected to the ER. Yeast lysates of wild-type and arf1-11 cells expressing Dcp2–9myc were prepared after a shift to 37°C for 1 h and separated into a 13,000 × g pellet (P13) and a corresponding supernatant (S13). The pellet was subjected to buoyant density centrifugation. A fraction of Dcp2–9myc cosedimented with the ER marker Sec61p in the P13 fraction and floated with Sec61p to the 0/40% sucrose interphase. Dcp2–9myc behaved similar in wild-type and arf1-11 lysates.