Figure 4.
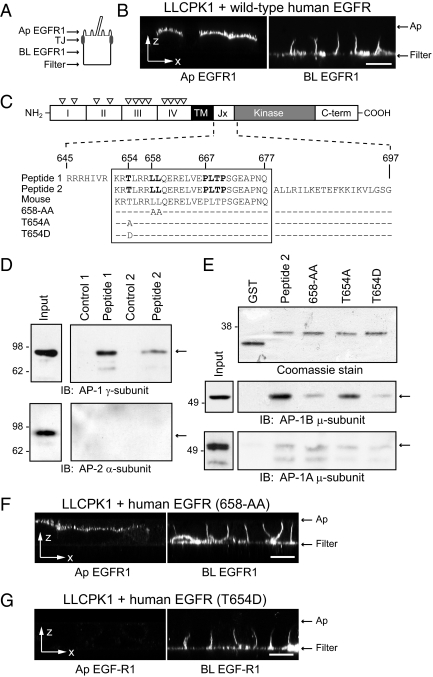
EGFR 658-LL basolateral sorting signal interacts with AP-1B. (A) Human-specific EGFR expression was evaluated by domain-specific EGFR1 staining of nonpermeabilized cells with well-formed tight junctions (TJ). (B) Horizontal x-z optical sections from LLCPK1 cells expressing wild-type human EGFR after domain-specific EGFR1 staining. (C) EGFR protein schematic highlights extracellular region with four subdomains (I–IV) and multiple N-glycosylation sites (▿), transmembrane (TM) domain, and cytoplasmic region with juxtamembrane (Jx), kinase catalytic, and carboxyl terminal (C-term) domains. Amino acid sequences of wild-type (peptide 1 and peptide 2) and mutant (T654A, T654D, and 658-AA) peptides used in pulldown assays are shown beneath the schematic. Critical residues involved in basolateral sorting are highlighted in bold (Hobert et al., 1997; He et al., 2002). Peptide sequences derived from human EGFR (Swiss-Prot: P00533.2) are precisely conserved in mouse EGFR (GenBank: AAA17899.1). (D) Peptides conjugated to Sepharose beads (peptide 1) or GST fusion protein attached to glutathione beads (peptide 2) were incubated with cell fractions enriched for AP complexes, and bound proteins were immunoblotted with AP-specific antibodies. Cell fractions were also incubated with empty Sepharose beads (Control 1) or affinity purified GST protein (Control 2). (E) Top: Coomassie-stained gel of affinity-purified GST fusion proteins. Bottom: GST fusion protein pulldown assays analyzed with a previously published AP-1B μ-subunit specific antibody (Fölsch et al., 1999). (F and G) Horizontal x-z optical sections from LLCPK1 cells expressing human EGFRs with 658-AA (F) or T654D (G) substitutions stained with domain-specific EGFR1. Scale bars, (B, F, and G) 5 μm.