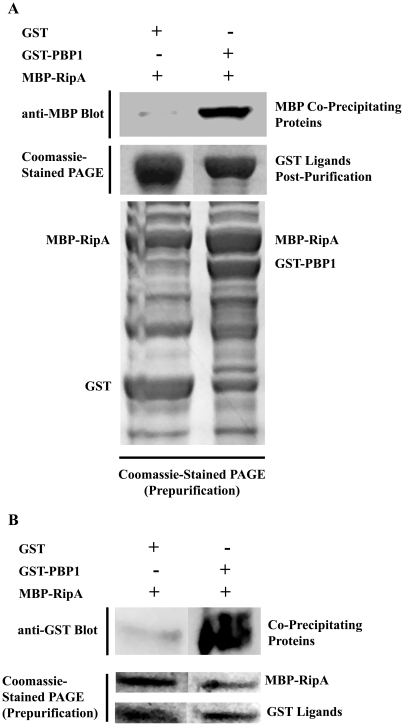
Figure 3. Recombinant PBP1 coprecipitates with RipA in vitro.
(A) Fusion proteins were co-expressed in E. coli and GST fusion proteins were purified directly from the lysate. Co-purifying MBP fusion proteins were detected by Western blotting using anti-MBP antibody (top panel). Unfused GST was used to test the specificity of the interaction. A Coomassie-stained PAGE gel containing lysates obtained prior to GST purification (bottom panel) and after GST purification (middle panel) is shown to demonstrate that similar amounts of proteins were available for pulldown. (B) Proteins were separately purified from E. coli, combined as indicated in equimolar amounts, incubated, then purified on amylose resin. Samples were taken before (middle and bottom panels) and after (top panel) MBP purification. A Coomassie-stained PAGE gel containing protein mixtures prior to MBP purification is shown to demonstrate that similar amounts of proteins were available for pulldown. Co-purifying GST fusion proteins were detected by Western blotting using anti-GST antibody. Unfused GST was used to test the specificity of the interaction.