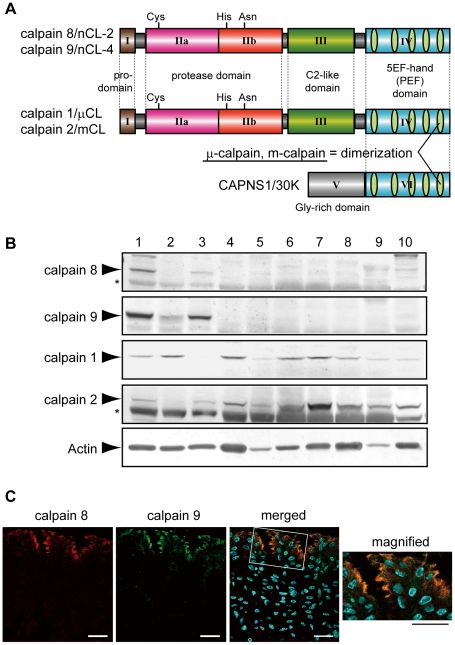
Figure 1. Calpains 8 and 9 showed the same tissue distribution and localization in the stomach.
(A) Schematic illustrations of mammalian calpains 1, 2, 8, and 9. Calpains 8 and 9 have a typical domain structure like calpains 1 and 2: a regulatory N-terminal pro-domain (domain I), protease domain (subdomains IIa and IIb, also called subdomains I and II), C2-like Ca2+/phospholipid-binding domain (domain III), and penta-EF-hand (PEF) domain (domain IV). CAPNS1 includes a Glycine-rich domain (domain V) and PEF domain (domain VI). Calpain 8 shows high similarity to calpain 2 over the whole molecule (amino acid identities of the full-length protein and of domains II and IV are 61.5%, 73.4%, and 51.8%, respectively). Calpain 1 (or 2) forms a heterodimer with CAPNS1 to be μ-calpain (or m-calpain). (B) Western blot analysis of mouse tissues using antibodies against calpain 8, calpain 9, calpain 1, calpain 2, and β-actin. Twenty micrograms of tissue homogenate was used for each lane except for β-actin, for which 2 µg of homogenate was used. Lanes: 1, gastric mucosa; 2, jejunum; 3, colon; 4, spleen; 5, liver; 6, kidney; 7, lung; 8, uterus; 9, heart; 10, brain. Asterisks indicate non-specific signals originating from the secondary antibody. (C) Double-immunostaining of the mouse stomach was performed using antibodies against calpain 8 (red) and calpain 9 (green). The right panel is a magnified view of the boxed area. Blue signals represent DAPI-stained nuclei. Bars, 50 µm.